Related Research Articles
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is the total mass of water in a substance at a given temperature and is mostly present in a definite (stoichiometric) ratio. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt, which is not directly bonded to the metal cation.

Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt is yellow, but the more familiar hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure.

Nickel(II) carbonate describes one or a mixture of inorganic compounds containing nickel and carbonate. From the industrial perspective, the most important nickel carbonate is basic nickel carbonate with the formula Ni4CO3(OH)6(H2O)4. Simpler carbonates, ones more likely encountered in the laboratory, are NiCO3 and its hexahydrate. All are paramagnetic green solids containing Ni2+ cations. The basic carbonate is an intermediate in the hydrometallurgical purification of nickel from its ores and is used in electroplating of nickel.

Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH)2. It is a lime-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.

Nickel(II) sulfate, or just nickel sulfate, usually refers to the inorganic compound with the formula NiSO4(H2O)6. This highly soluble blue green coloured salt is a common source of the Ni2+ ion for electroplating.

Nickel(II) bromide is the name for the inorganic compounds with the chemical formula NiBr2(H2O)x. The value of x can be 0 for the anhydrous material, as well as 2, 3, or 6 for the three known hydrate forms. The anhydrous material is a yellow-brown solid which dissolves in water to give blue-green hexahydrate (see picture).

Nickel(II) acetate is the name for the coordination compounds with the formula Ni(CH3CO2)2·x H2O where x can be 0, 2, and 4. The green tetrahydrate Ni(CH3CO2)2·4 H2O is most common. It is used for electroplating.

Magnesium oxalate is an organic compound comprising a magnesium cation with a 2+ charge bonded to an oxalate anion. It has the chemical formula MgC2O4. Magnesium oxalate is a white solid that comes in two forms: an anhydrous form and a dihydrate form where two water molecules are complexed with the structure. Both forms are practically insoluble in water and are insoluble in organic solutions.
Chromium(II) oxalate is an inorganic compound with the chemical formula CrC2O4.
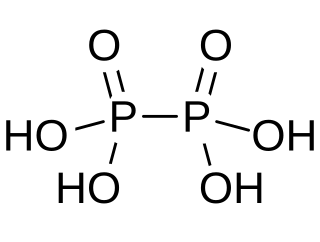
Hypophosphoric acid is a mineral acid with the formula H4P2O6, with phosphorus in a formal oxidation state of +4. In the solid state it is present as the dihydrate, H4P2O6·2H2O. In hypophosphoric acid the phosphorus atoms are identical and joined directly with a P−P bond. Isohypophosphoric acid is a structural isomer of hypophosphoric acid in which one phosphorus has a hydrogen directedly bonded to it and that phosphorus atom is linked to the other one by an oxygen bridge to give a phosphorous acid/phosphoric acid mixed anhydride. The two phosphorus atoms are in the +3 and +5 oxidation states, respectively.

Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds formed from Bi2O3, N2O5 and H2O. Other names for bismuth oxynitrate include bismuth subnitrate and bismuthyl nitrate. In older texts bismuth oxynitrate is often simply described as BiONO3 or basic bismuth nitrate. Bismuth oxynitrate was once called magisterium bismuti or bismutum subnitricum, and was used as a white pigment, in beauty care, and as a gentle disinfectant for internal and external use. It is also used to form Dragendorff's reagent, which is used as a TLC stain.

Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.
Nickel compounds are chemical compounds containing the element nickel which is a member of the group 10 of the periodic table. Most compounds in the group have an oxidation state of +2. Nickel is classified as a transition metal with nickel(II) having much chemical behaviour in common with iron(II) and cobalt(II). Many salts of nickel(II) are isomorphous with salts of magnesium due to the ionic radii of the cations being almost the same. Nickel forms many coordination complexes. Nickel tetracarbonyl was the first pure metal carbonyl produced, and is unusual in its volatility. Metalloproteins containing nickel are found in biological systems.
The oxalatonickelates are a class of compounds that contain nickel complexed by oxalate groups. They form a series of double salts, and include clusters with multiple nickel atoms. Since oxalate functions as a bidentate ligand it can satisfy two coordinate positions around the nickel atom, or it can bridge two nickel atoms together.

Chevreul's salt (copper(I,II) sulfite dihydrate, Cu2SO3•CuSO3•2H2O or Cu3(SO3)2•2H2O), is a copper salt which was prepared for the first time by a French chemist Michel Eugène Chevreul in 1812. Its unusual property is that it contains copper in both of its common oxidation states, making it a mixed-valence complex. It is insoluble in water and stable in air. What was known as Rogojski's salt is a mixture of Chevreul's salt and metallic copper.
Nickel is one of the metals that can form Tutton's salts. The singly charged ion can be any of the full range of potassium, rubidium, cesium, ammonium (), or thallium. As a mineral the ammonium nickel salt, (NH4)2Ni(SO4)2 · 6 H2O, can be called nickelboussingaultite. With sodium, the double sulfate is nickelblödite Na2Ni(SO4)2 · 4 H2O from the blödite family. Nickel can be substituted by other divalent metals of similar sized to make mixtures that crystallise in the same form.

The Nickel oxyacid salts are a class of chemical compounds of nickel with an oxyacid. The compounds include a number of minerals and industrially important nickel compounds.
The nickel organic acid salts are organic acid salts of nickel. In many of these the ionised organic acid acts as a ligand.

Neodymium nitrate is a chemical compound with the formula Nd(NO3)3. It is typically encountered as the hexahydrate, Nd(NO3)3·6H2O, which is more accurately formulated as [Nd(NO3)3(H2O)4].2H2O to reflect the crystal structure. It decomposes to NdONO3 at elevated temperature. This water-soluble salt finds use in fabrication of perovskite (CaTiO3) based solid oxide fuel cells, synthesis of Nd3+ doped vanadium pentoxide (V2O5) nanostructure for potential usage in supercapacitors and as a catalyst for Friedlander synthesis of surface modified quinolones for application in medicinal chemistry.
Manganese oxalate is a chemical compound, a salt of manganese and oxalic acid with the chemical formula MnC
2O
4. The compound creates light pink crystals, does not dissolve in water, and forms crystalline hydrates. It occurs naturally as the mineral Lindbergite.
References
- 1 2 3 4 Forster, Paul M.; Cheetham, Anthony K. (1 February 2002). "Open-Framework Nickel Succinate, [Ni7(C4H4O4)6(OH)2(H2O)2]⋅2 H2O: A New Hybrid Material with Three-Dimensional Ni−O−Ni Connectivity". Angewandte Chemie International Edition. 41 (3): 457–459. doi:10.1002/1521-3773(20020201)41:3<457::AID-ANIE457>3.0.CO;2-W. PMID 12491377.
- ↑ Bear, John L.; Lin, Chin-Tung (June 1968). "Kinetics of formation of nickel malonate and nickel succinate complexes". The Journal of Physical Chemistry. 72 (6): 2026–2029. doi:10.1021/j100852a027.
- 1 2 Mohamed, Mohamed A; Galwey, Andrew K; Halawy, Samih A (December 1998). "Kinetic and thermodynamic studies of the non-isothermal decompositions of nickel malonate dihydrate and nickel hydrogen malonate dihydrate". Thermochimica Acta. 323 (1–2): 27–36. doi:10.1016/S0040-6031(98)00492-4.
- 1 2 Abd El-Salaam, K.M.; Halawani, K.H.; Fakiha, S.A. (August 1992). "Kinetic analysis of non-isothermal decomposition of Fe2+, Co2+, Ni2+ and Cu2+ succinate complexes in a nitrogen atmosphere". Thermochimica Acta. 204 (2): 311–320. doi:10.1016/0040-6031(92)85235-N.
- 1 2 Forster, Paul M.; Cheetham, Anthony K. (1 February 2002). "Open-Framework Nickel Succinate, [Ni7(C4H4O4)6(OH)2(H2O)2]⋅2 H2O: A New Hybrid Material with Three-Dimensional Ni−O−Ni Connectivity". Angewandte Chemie International Edition. 41 (3): 457–459. doi:10.1002/1521-3773(20020201)41:3<457::AID-ANIE457>3.0.CO;2-W. PMID 12491377.