
Niacinamide or Nicotinamide (NAM) is a form of vitamin B3 found in food and used as a dietary supplement and medication. As a supplement, it is used by mouth to prevent and treat pellagra (niacin deficiency). While nicotinic acid (niacin) may be used for this purpose, niacinamide has the benefit of not causing skin flushing. As a cream, it is used to treat acne. It is a water-soluble vitamin. Niacinamide is the supplement name while Nicotinamide (NAM) is the scientific name.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.
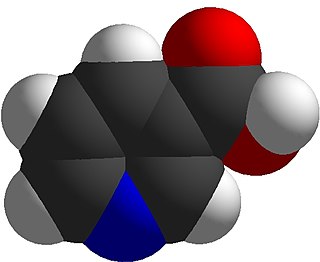
Niacin, also known as nicotinic acid, is an organic compound and a form of vitamin B3, an essential human nutrient. It can be manufactured by plants and animals from the amino acid tryptophan. Niacin is obtained in the diet from a variety of whole and processed foods, with highest contents in fortified packaged foods, meat, poultry, red fish such as tuna and salmon, lesser amounts in nuts, legumes and seeds. Niacin as a dietary supplement is used to treat pellagra, a disease caused by niacin deficiency. Signs and symptoms of pellagra include skin and mouth lesions, anemia, headaches, and tiredness. Many countries mandate its addition to wheat flour or other food grains, thereby reducing the risk of pellagra.
In organic chemistry, a nitrile is any organic compound that has a −C≡N functional group. The prefix cyano- is used interchangeably with the term nitrile in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons.

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.

Picolinic acid is an organic compound with the formula C5H4N(CO2H). It is a derivative of pyridine with a carboxylic acid (COOH) substituent at the 2-position. It is an isomer of nicotinic acid and isonicotinic acid, which have the carboxyl side chain at the 3- and 4-position, respectively. It is a white solid that is soluble in water.
Nitrile hydratases are mononuclear iron or non-corrinoid cobalt enzymes that catalyse the hydration of diverse nitriles to their corresponding amides
The Chichibabin pyridine synthesis is a method for synthesizing pyridine rings. The reaction involves the condensation reaction of aldehydes, ketones, α,β-Unsaturated carbonyl compounds, or any combination of the above, with ammonia. It was reported by Aleksei Chichibabin in 1924. Methyl-substituted pyridines, which show widespread uses among multiple fields of applied chemistry, are prepared by this methodology.
In enzymology, an aliphatic nitrilase also known as aliphatic nitrile aminohydrolase (EC 3.5.5.7) is an enzyme that catalyzes the hydrolysis of nitriles to carboxylic acids:
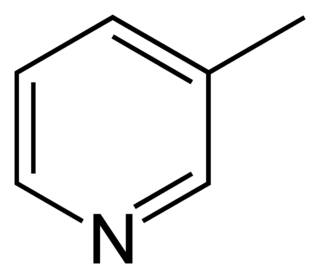
3-Methylpyridine or 3-picoline, is an organic compound with formula 3-CH3C5H4N. It is one of three positional isomers of methylpyridine, whose structures vary according to where the methyl group is attached around the pyridine ring. This colorless liquid is a precursor to pyridine derivatives that have applications in the pharmaceutical and agricultural industries. Like pyridine, 3-methylpyridine is a colorless liquid with a strong odor and is classified as a weak base.
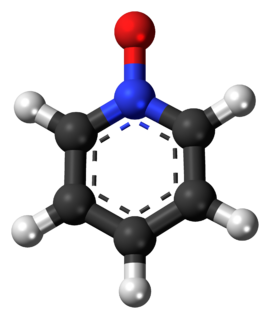
Pyridine-N-oxide is the heterocyclic compound with the formula C5H5NO. This colourless, hygroscopic solid is the product of the oxidation of pyridine. It was originally prepared using peroxyacids as the oxidising agent. The compound is used infrequently as an oxidizing reagent in organic synthesis.
4-Methylpyridine is the organic compound with the formula CH3C5H4N. It is one of the three isomers of methylpyridine. This pungent liquid is a building block for the synthesis of other heterocyclic compounds. Its conjugate acid, the 4-methylpyridinium ion, has a pKa of 5.98, about 0.7 units above that of pyridine itself.

3-Aminopyridine is an aminopyridine. It is a colorless solid.

A-366,833 is a drug developed by Abbott, which acts as an agonist at neural nicotinic acetylcholine receptors selective for the α4β2 subtype, and has been researched for use as an analgesic, although it has not passed clinical trials. Its structure has a nicotinonitrile (3-cyanopyridine) core bound through C5 to the N6 of (1R,5S)-3,6-diazabicyclo[3.2.0]heptane.
In organic chemistry, ammoxidation is a process for the production of nitriles using ammonia and oxygen. It is sometimes called the SOHIO process, acknowledging that ammoxidation was developed at Standard Oil of Ohio. The usual substrates are alkenes. Several million tons of acrylonitrile are produced in this way annually:

2-Methylglutaronitrile is the organic compound with the formula NCCH2CH2CH(CH3)CN. This dinitrile is obtained in the large-scale synthesis of adiponitrile. It is a colorless liquid with an unpleasant odor. It is the starting compound for the vitamin nicotinamide and for the diester dimethyl-2-methylglutarate and the ester amide methyl 5-(dimethylamino)-2-methyl-5-oxopentanoate, which are promoted as green solvents. 2-Methylglutaronitrile is chiral but is mainly encountered as the racemate.

Cyanoethylation is a process for the attachment of CH2CH2CN group to another organic substrate. The method is used in the synthesis of organic compounds.
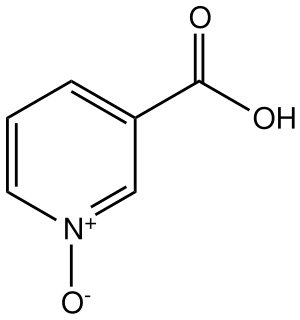
Nicotinic acid N-oxide is an organic compound with the formula (HO2C)C5H4NO. It is the N-oxide of nicotinic acid ((HO2C)C5H4N). It is prepared by oxidation of nicotinic acid or the hydrolysis of 3-cyanopyridine N-oxide. The compound is a precursor to the popular drugs niflumic acid and pranoprofen.

5-Ethyl-2-methylpyridine is an organic compound with the formula (C2H5)(CH3)C5H3N. One of several isomeric pyridines with this formula, this derivative is of interest because it is efficiently prepared from simple reagents and it is a convenient precursor to nicotinic acid, a form of vitamin B3. 5-Ethyl-2-methylpyridine is a colorless liquid.

3-Chlorobenzonitrile is an organic compound with the chemical formula ClC6H4CN. It is one of the isomers of chlorobenzonitrile.
















