Components
Stem
Until 2021, the stem -mab was used for all monoclonal antibodies as well as for their fragments, as long as at least one variable domain (the domain that contains the target binding structure) was included. [9] This is the case for antigen-binding fragments [10] and single-chain variable fragments, [11] among other artificial proteins.
The new scheme, published in November 2021, divides antibodies into four groups: Group 1 uses the stem -tug for full-length unmodified immunoglobulins, those that might occur as such in the immune system. Group 2 has the stem -bart for full-length antibodies artificial, which contain one or more engineered regions (at least one point mutation). Group 3 uses -mig for multi-immunoglobulins of any length, comprising bispecific and multispecific monoclonal antibodies. Finally, group 4 assigns the stem -ment for monospecific antibody fragments without an Fc region. [1] [2]
Other antibody parts (such as Fc regions) and antibody mimetics use different naming schemes.
Substem for source

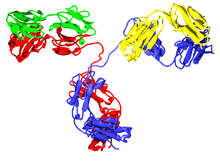
Human parts are shown in brown, non-human parts in blue. The variable domains are the boxes on top of each antibody; the CDRs within these domains are represented as triple loops.
For antibodies named until early 2017, the substem preceding the stem denotes the animal from which the antibody was obtained. [7] The first monoclonal antibodies were produced in mice (substem -o-, yielding the ending -omab; usually Mus musculus , the house mouse) or other non-human organisms. Neither INN nor USAN has ever been requested for antibodies from rats (theoretically -a-), hamsters (-e-) and primates (-i-). [9]
These non-human antibodies are recognized as foreign by the human immune system and may be rapidly cleared from the body, provoke an allergic reaction, or both. [12] [13] To avoid this, parts of the antibody can be replaced with human amino acid sequences, or pure human antibodies can be engineered. If the constant region is replaced with the human form, the antibody is termed chimeric and the substem used was -xi-. Part of the variable regions may also be substituted, in which case it is called humanized and -zu- was used; typically, everything is replaced except the complementarity-determining regions (CDRs), the three loops of amino acid sequences at the outside of each variable region that bind to the target structure; although some other residues may have to remain non-human in order to achieve good binding. Partly chimeric and partly humanized antibodies used -xizu-. These three substems did not indicate the foreign species used for production. Thus, the human/mouse chimeric antibody basiliximab ends in -ximab just as does the human/macaque antibody gomiliximab. Purely human antibodies used -u-. [3]
Rat/mouse hybrid antibodies can be engineered with binding sites for two different antigens. These drugs, termed trifunctional antibodies , had the substem -axo-. [14]
Newer antibody names omit this part of the name. [8]
Substem for target
The substem preceding the source of the antibody refers to the medicine's target. Examples of targets are tumors, organ systems like the circulatory system, or infectious agents like bacteria or viruses. The term target does not imply what sort of action the antibody exerts. Therapeutic, prophylactic and diagnostic agents are not distinguished by this nomenclature.
In the naming scheme as originally developed, these substems mostly consist of a consonant, a vowel, then another consonant. The final letter may be dropped if the resulting name would be difficult to pronounce otherwise. Examples include -ci(r)- for the circulatory system, -li(m)- for the immune system (lim stands for lymphocyte) and -ne(r)- for the nervous system. The final letter is usually omitted if the following source substem begins with a consonant (such as -zu- or -xi-), but not all target substems are used in their shortened form. -mul-, for example, is never reduced to -mu- because no chimeric or humanized antibodies targeting the musculoskeletal system ever received an INN. Combination of target and source substems resulted in endings like -limumab (immune system, human) or -ciximab (circulatory system, chimeric, consonant r dropped). [7]
New and shorter target substems were adopted in 2009. They mostly consist of a consonant, plus a vowel which is omitted if the source substem begins with a vowel. For example, human antibodies targeting the immune system receive names ending in -lumab instead of the old -limumab. Some endings like -ciximab remained unchanged. [3] The old system employed seven different substems for tumor targets, depending on the type of tumor. Because many antibodies are investigated for several tumor types, the new convention only has -t(u)-. [7]
With the source substem being discontinued in 2017, the need for dropping the target substem's final vowel disappeared. [8]
Prefix
The prefix carries no special meaning. It should be unique for each medicine and contribute to a well-sounding name. [3] This means that antibodies with the same source and target substems are only distinguished by their prefix. Even antibodies targeting exactly the same structure are differently prefixed, such as the adalimumab and golimumab, both of which are TNF inhibitors but differ in their chemical structure. [15] [16]
Additional words
A second word following the name of the antibody indicates that another substance is attached, [3] which is done for several reasons.
- An antibody can be PEGylated (attached to molecules of polyethylene glycol) to slow down its degradation by enzymes and to decrease its immunogenicity; [17] this is shown by the word pegol as in alacizumab pegol . [18]
- A cytotoxic agent can be linked to an anti-tumor antibody for drug targeting purposes. The word vedotin, for example, stands for monomethyl auristatin E which is toxic by itself but predominantly affects cancer cells if used in conjugates like glembatumumab vedotin. [19]
- A chelator for binding a radioisotope can be attached. Pendetide, a derivative of pentetic acid, is used for example in capromab pendetide to chelate indium-111. [20] If the drug contains a radioisotope, the name of the isotope precedes the name of the antibody. [3] Consequently, indium (111In) capromab pendetide is the name for the above example including indium-111. [20]
Overview
| Prefix | Target substem | Source substem (until 2017) | Stem | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ~1993 | 2009–2017 | 2017–2021 | from 2021 | meaning | meaning | old | from 2021 | meaning | |||
| variable | — | -ami- | -ami- | -ami- | serum amyloid protein (SAP)/amyloidosis (pre-substem) | -a- | rat | -mab | -bart | artificial antibody | |
| -anibi- | — | — | — | angiogenesis (inhibitor) | -e- | hamster | -ment | fragment (derived from a variable domain) | |||
| -ba(c)- | -b(a)- | -ba- | -ba- | bacterial | -i- | primate | -mig | multi-immunoglobulin (e.g. BsMAb) | |||
| -ci(r)- | -c(i)- | -ci- | -ci- | cardiovascular | -o- | mouse | -tug | unmodified immunoglobulin | |||
| -d(e)- | -d(e)- [lower-alpha 1] | -de- [lower-alpha 1] | -de- | endocrine | -u- | human | |||||
| — | — | -eni- [lower-alpha 2] | -eni- | enzyme inhibition | -xi- | chimeric (human/foreign) | |||||
| -fung- | -f(u)- | -fung- | -fung- | fungal | -zu- | humanized | |||||
| -gr(o)- | -gros- | -gros- | -gro- [lower-alpha 3] | skeletal muscle mass related growth factors and receptors (pre-substem) | -xizu- | chimeric/humanized hybrid | |||||
| -ki(n)- | -k(i)- | -ki- | -ki- | formerly: interleukin; from 2020: cytokine and cytokine receptor | -axo- | rat/mouse hybrid (see trifunctional antibody ) | |||||
| -les- | — | — | — | inflammatory lesions [25] | |||||||
| -li(m)- | -l(i)- | -li- | -ler- | immunomodulating | allergen | -vet- | veterinary use (pre-substem) [lower-alpha 4] | ||||
| -pru- | immunosuppressive | ||||||||||
| -sto- | immunostimulatory | ||||||||||
| -mul- | — | — | — | musculoskeletal system [26] | |||||||
| -ne(u)(r)- | -n(e)- | -ne- | -ne- | neural (nervous system) | |||||||
| -os- | -s(o)- | -os- | -os- | bone | |||||||
| -co(l)- | -t(u)- | -ta- | -ta- | colonic tumor | tumor | ||||||
| -go(t)- | testicular tumor | ||||||||||
| -go(v)- | ovarian tumor | ||||||||||
| -ma(r)- | mammary tumor | ||||||||||
| -me(l)- | melanoma | ||||||||||
| -pr(o)- | prostate tumor | ||||||||||
| -tu(m)- | miscellaneous tumor | ||||||||||
| -toxa- | -tox(a)- | -toxa- | -toxa- | toxin | |||||||
| — | — | -vet- | -vet- | veterinary use (pre-stem) [lower-alpha 4] | |||||||
| -vi(r)- | -v(i)- | -vi- | -vi- | viral | |||||||
- 1 2 not mentioned in documents of the 2010s
- ↑ introduced between 2017 and 2021
- ↑ changed in 2019 from -gros- to -gro- to avoid naming conflicts with -os-
- 1 2 can be inserted between target substem and stem, as in lo-ki-vet-mab (This part of the name was originally listed under the source substems and moved to the target substems when the former were dropped in 2017. [8] )
