Therapeutic, diagnostic and preventive monoclonal antibodies are clones of a single parent cell. When used as drugs, the International Nonproprietary Names (INNs) end in -mab. The remaining syllables of the INNs, as well as the column Source, are explained in Nomenclature of monoclonal antibodies .
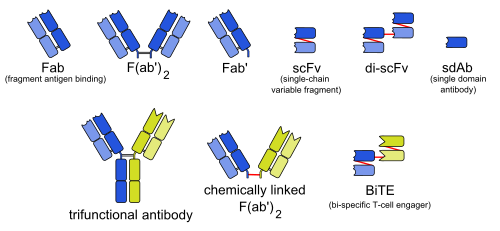
The abbreviations in the column Type are as follows:
- mab: whole monoclonal antibody
- Fab: fragment, antigen-binding (one arm)
- F(ab')2: fragment, antigen-binding, including hinge region (both arms)
- Fab': fragment, antigen-binding, including hinge region (one arm)
- Variable fragments:
- scFv: single-chain variable fragment
- di-scFv: dimeric single-chain variable fragment
- sdAb: single-domain antibody
- BsAb: bispecific monoclonal antibody:
- 3funct: trifunctional antibody
- BiTE: bi-specific T-cell engager
This list of over 500 monoclonal antibodies includes approved and investigational drugs as well as drugs that have been withdrawn from market; consequently, the column Use does not necessarily indicate clinical usage. See the list of FDA-approved therapeutic monoclonal antibodies in the monoclonal antibody therapy page.
| Name | Brand name | Type | Source | Target | Approved | Use |
|---|---|---|---|---|---|---|
| 3F8 | mab | mouse | GD2 ganglioside | neuroblastoma | ||
| Abagovomab [1] | mab | mouse | CA-125 (imitation) | ovarian cancer | ||
| Abciximab [2] | ReoPro | Fab | chimeric | CD41 (integrin alpha-IIb) | Y [3] | platelet aggregation inhibitor |
| Abituzumab [4] | mab | humanized | CD51 | cancer | ||
| Abrezekimab [5] | Fab | humanized | IL-13 | |||
| Abrilumab [6] | mab | human | integrin α4 β7 | inflammatory bowel disease, ulcerative colitis, Crohn's disease | ||
| Actoxumab [6] | mab | human | Clostridioides difficile | Clostridioides difficile colitis | ||
| Adalimumab [7] | Humira | mab | human | TNF-α | Y [8] | rheumatoid arthritis, Crohn's disease, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, juvenile idiopathic arthritis, hemolytic disease of the newborn |
| Adecatumumab [9] | mab | human | EpCAM | prostate and breast cancer | ||
| Aducanumab [10] | Aduhelm | mab | human | Amyloid beta | Y [11] | Alzheimer's disease |
| Afasevikumab [12] | mab | human | IL-17A, IL-17F | multiple sclerosis | ||
| Afelimomab [2] | F(ab')2 | mouse | TNF-α | sepsis | ||
| Alacizumab pegol [13] | F(ab')2 | humanized | VEGFR2 | cancer | ||
| Alemtuzumab [14] | Lemtrada, Campath | mab | humanized | CD52 | Y | multiple sclerosis |
| Alirocumab [15] | Praluent | mab | human | PCSK9 | Y | hypercholesterolemia |
| Altumomab pentetate | Hybri-ceaker | mab | mouse | Carcinoembryonic antigen (CEA) | colorectal cancer (diagnosis) | |
| Amatuximab [16] | mab | chimeric | mesothelin | cancer | ||
| Amivantamab | Rybrevant | BsAb | human | Epidermal growth factor receptor (EGFR), cMet | Y | non-small cell lung cancer |
| Anatumomab mafenatox [17] | Fab | mouse | Tumor-associated glycoprotein 72 (TAG-72) | non-small cell lung cancer | ||
| Andecaliximab [18] | mab | chimeric | gelatinase B | gastric cancer or gastroesophageal junction adenocarcinoma | ||
| Anetumab ravtansine [4] | mab | human | mesothelin (MSLN) | cancer | ||
| Anifrolumab [4] | Saphnelo | mab | human | IFN-α/β receptor | Y [19] | systemic lupus erythematosus |
| Ansuvimab | Ebanga | mab | human | Ebola virus glycoprotein | Y | treatment of Zaire ebolavirus (Ebola virus) |
| Anrukinzumab [13] (= IMA-638) [20] | mab | humanized | IL-13 | asthma | ||
| Apolizumab [21] | mab | humanized | HLA-DR | hematological cancers | ||
| Aprutumab ixadotin [22] | mab | human | FGFR2 | |||
| Arcitumomab [23] | CEA-Scan | Fab' | mouse | Carcinoembryonic antigen (CEA) | gastrointestinal cancers (diagnosis) | |
| Ascrinvacumab [12] | mab | human | activin receptor-like kinase 1 | cancer | ||
| Aselizumab [24] | mab | humanized | L-selectin (CD62L) | severely injured patients | ||
| Atezolizumab [25] | Tecentriq | mab | humanized | PD-L1 | Y | cancer |
| Atidortoxumab [26] | mab | human | Staphylococcus aureus alpha toxin | |||
| Atinumab [16] | mab | human | RTN4 | |||
| Atoltivimab | mab | human | Ebola virus glycoprotein | part of Atoltivimab/maftivimab/odesivimab for treatment of Zaire ebolavirus (Ebola virus) | ||
| Atoltivimab/maftivimab/odesivimab | Inmazeb | mab | human | Y | treatment of Zaire ebolavirus (Ebola virus) | |
| Atorolimumab [2] | mab | human | Rhesus factor | hemolytic disease of the newborn [ citation needed ] | ||
| Avelumab [12] | Bavencio | mab | human | PD-L1 | Y | cancer |
| Axatilimab [27] | Niktimvo | mab | humanized | CSF1R | Y | chronic graft-versus-host disease |
| Azintuxizumab vedotin [28] | mab | chimeric/ humanized | CD319 | cancer | ||
| Bamlanivimab [29] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | US emergency use authorization (EUA) when used with etesevimab [30] | COVID-19 | |
| Bapineuzumab [31] | mab | humanized | β-amyloid | Alzheimer's disease | ||
| Basiliximab [32] | Simulect | mab | chimeric | CD25 (α chain of IL-2 receptor) | Y | prevention of organ transplant rejections |
| Bavituximab [1] | mab | chimeric | phosphatidylserine | cancer, viral infections | ||
| BCD-100 | human | PD-1 | melanoma | |||
| Bebtelovimab [33] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | US emergency use authorization (EUA) [34] | COVID-19 | |
| Bectumomab [32] | LymphoScan | Fab' | mouse | CD22 | non-Hodgkin's lymphoma (detection) | |
| Bedinvetmab [35] | Librela [36] | mab | veterinary | nerve growth factor (NGF) [36] | Y | pain associated with osteoarthritis in dogs [36] |
| Begelomab [6] | mab | mouse | DPP4 | |||
| Belantamab mafodotin [5] | Blenrep | mab | humanized | B-cell maturation antigen (BCMA) | Y (Withdrawn) | relapsed or refractory multiple myeloma |
| Belimumab [37] | Benlysta | mab | human | B-cell activating factor (BAFF) | Y | systemic lupus erythematosus without renal or CNS involvement |
| Bemarituzumab [26] | mab | humanized | FGFR2 | gastric cancer or gastroesophageal junction adenocarcinoma | ||
| Benralizumab [38] | Fasenra | mab | humanized | CD125 | Y | asthma |
| Berlimatoxumab [26] | mab | human | Staphylococcus aureus bi-component leukocidin | |||
| Bermekimab [32] | Xilonix | mab | human | IL-1α | colorectal cancer | |
| Bersanlimab [5] | mab | human | ICAM-1 | |||
| Bertilimumab [24] | mab | human | CCL11 (eotaxin-1) | severe allergic disorders | ||
| Besilesomab [39] | Scintimun | mab | mouse | Carcinoembryonic antigen (CEA)-related antigen | inflammatory lesions and metastases (detection) | |
| Bevacizumab [14] | Avastin | mab | humanized | VEGF-A | Y | metastatic cancer, retinopathy of prematurity |
| Bezlotoxumab [32] | Zinplava | mab | human | Clostridioides difficile | Y | Clostridioides difficile colitis |
| Biciromab [32] | FibriScint | Fab' | mouse | fibrin II, beta chain | thromboembolism (diagnosis) | |
| Bimagrumab [40] | mab | human | ACVR2B | myostatin inhibitor | ||
| Bimekizumab [10] | Bimzelx | mab | humanized | IL-17A, IL-17F, IL-17AF | Y | psoriasis |
| Birtamimab | mab | chimeric | serum amyloid A protein | amyloidosis | ||
| Bivatuzumab [32] | mab | humanized | CD44 v6 | squamous cell carcinoma | ||
| Bleselumab [12] | mab | human | CD40 | organ transplant rejection | ||
| Blinatumomab [32] | Blincyto | BiTE | mouse | CD19 | Y | pre-B Acute lymphoblastic leukemia (ALL) (CD19+) |
| Blontuvetmab [41] | Blontress | mab | veterinary | CD20 | ||
| Blosozumab [42] | mab | humanized | SOST | osteoporosis | ||
| Bococizumab [32] | mab | humanized | PCSK9 | dyslipidemia | ||
| Brazikumab [18] | mab | human | IL-23 | Crohn's disease | ||
| Brentuximab vedotin [32] | Adcentris | mab | chimeric | CD30 (TNFRSF8) | Y | Hodgkin's lymphoma, anaplastic large-cell lymphoma |
| Briakinumab [32] | mab | human | IL-12, IL-23 | psoriasis, rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis | ||
| Brodalumab [42] | Siliq | mab | human | IL-17 | Y | Plaque psoriasis |
| Brolucizumab [25] | Beovu | scFv | humanized | vascular endothelial growth factor A (VEGFA) | Y | wet age-related macular degeneration |
| Brontictuzumab [6] | mab | humanized | Notch 1 | cancer | ||
| Burosumab [18] | Crysvita | mab | human | FGF 23 | Y | X-linked hypophosphatemia |
| Cabiralizumab [41] | mab | humanized | CSF1R | metastatic pancreatic cancer | ||
| Camidanlumab tesirine [26] | mab | human | CD25 (α chain of IL-2 receptor) | B-cell Hodgkin's lymphoma, non-Hodgkin lymphoma, acute lymphoblastic leukemia, acute myeloid leukemia | ||
| Camrelizumab [22] | mab | humanized | PD-1 | hepatocellular carcinoma | ||
| Canakinumab [43] | Ilaris | mab | human | IL-1 | Y | cryopyrin-associated periodic syndrome, Yao's Syndrome, Adult Onset Still's Disease |
| Cantuzumab mertansine [42] | mab | humanized | CanAg (a glycoform of MUC1) | colorectal cancer etc. | ||
| Cantuzumab ravtansine [42] | mab | humanized | CanAg (a glycoform of MUC1) | cancers | ||
| Caplacizumab [44] | Cablivi | sdAb | humanized | VWF | Y | thrombotic thrombocytopenic purpura, thrombosis |
| Casirivimab [29] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Yes when used with imdevimab [45] [46] | COVID-19 | |
| Capromab [32] | Prostascint | mab | mouse | Glutamate carboxypeptidase II | Y | prostate cancer (detection) |
| Carlumab [16] | mab | human | MCP-1 | oncology/immune indications | ||
| Carotuximab [41] | mab | chimeric | endoglin | angiosarcoma | ||
| Catumaxomab [31] | Removab | 3funct | rat/mouse hybrid | EpCAM, CD3 | Y | ovarian cancer, malignant ascites, gastric cancer |
| cBR96-doxorubicin immunoconjugate | mab | humanized | Lewis-Y antigen | cancer | ||
| Cedelizumab [47] | mab | humanized | CD4 | prevention of organ transplant rejections, treatment of autoimmune diseases | ||
| Cemiplimab [32] | Libtayo | mab | human | PD-1 | Y | cutaneous squamous cell carcinoma |
| Cergutuzumab amunaleukin [12] | mab | humanized | IL-2 | cancer | ||
| Certolizumab pegol [43] | Cimzia | Fab' | humanized | TNF-α | Y | Crohn's disease, rheumatoid arthritis, axial spondyloarthritis, psoriasis arthritis |
| Cetrelimab [5] | mab | human | PD-1 | cancer | ||
| Cetuximab [32] | Erbitux | mab | chimeric | Epidermal growth factor receptor (EGFR) | Y | metastatic colorectal cancer and head and neck cancer |
| Cibisatamab [5] | mab | humanized | CEACAM5 | cancer | ||
| Cilgavimab [29] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | US emergency use authorization (EUA) when used with tixagevimab [48] | COVID-19 | |
| Cirmtuzumab [32] | humanized | ROR1 | chronic lymphocytic leukemia | |||
| Citatuzumab bogatox [49] | Fab | humanized | EpCAM | ovarian cancer and other solid tumors | ||
| Cixutumumab [32] | mab | human | IGF-1 receptor (CD221) | solid tumors | ||
| Clazakizumab [50] | mab | humanized | IL-6 | rheumatoid arthritis | ||
| Clenoliximab [32] | mab | chimeric | CD4 | rheumatoid arthritis | ||
| Clesrovimab [51] | Enflonsia | mab | human | respiratory syncytial virus F protein | Y | respiratory syncytial virus (prevention) |
| Clivatuzumab tetraxetan [32] | hPAM4-Cide | mab | humanized | MUC1 | pancreatic cancer | |
| Codrituzumab [4] | mab | humanized | glypican 3 | cancer | ||
| Cofetuzumab pelidotin [26] | mab | humanized | PTK7 | cancer | ||
| Coltuximab ravtansine [4] | mab | chimeric | CD19 | cancer | ||
| Conatumumab [49] | mab | human | TRAIL-R2 | cancer | ||
| Concizumab [52] | Alhemo | mab | humanized | tissue factor pathway inhibitor (TFPI) | Y | bleeding with hemophilia |
| Cosfroviximab [28] | ZMapp | mab | chimeric | ebolavirus glycoprotein | Ebola virus | |
| Cosibelimab [27] | Unloxcyt [53] | mab | human | Programmed death ligand-1 (PD-L1) | Y | cutaneous squamous cell carcinoma |
| Crenezumab [32] | mab | humanized | β-amyloid (1-40 and 1-42) | Alzheimer's disease | ||
| Crizanlizumab [22] | Adakveo | mab | humanized | selectin P | Y | sickle-cell disease |
| Crotedumab [41] | mab | human | glucagon receptor (GCGR) | diabetes | ||
| Crovalimab [54] | Piasky | mab | humanized | C5 | Y | paroxysmal nocturnal hemoglobinuria |
| CR6261 | mab | human | Hemagglutinin (influenza) | infectious disease/influenza A | ||
| Cusatuzumab [5] | mab | humanized | CD70 | cancer | ||
| Dacetuzumab [13] | mab | humanized | CD40 | hematologic cancers | ||
| Daclizumab [55] | Zenapax | mab | humanized | CD25 (α chain of IL-2 receptor) | Y | prevention of organ transplant rejections, multiple sclerosis |
| Dalotuzumab | mab | humanized | IGF-1 receptor (CD221) | cancer etc. | ||
| Dapirolizumab pegol [56] | mab | humanized | CD154 (CD40L) | |||
| Daratumumab [57] | Darzalex | mab | human | CD38 | Y [58] | multiple myeloma |
| Dectrekumab [25] | mab | human | IL-13 | |||
| Demcizumab | mab | humanized | DLL4 | cancer | ||
| Denintuzumab mafodotin [6] | mab | humanized | CD19 | cancer | ||
| Denosumab [59] | Prolia | mab | human | RANKL | Y | osteoporosis, bone metastases etc. |
| Depatuxizumab mafodotin [22] | mab | chimeric/ humanized | EGFR | glioblastoma | ||
| Derlotuximab biotin | mab | chimeric | histone complex | recurrent glioblastoma multiforme | ||
| Detumomab | mab | mouse | B-lymphoma cell | lymphoma | ||
| Dezamizumab [22] | mab | humanized | serum amyloid P component | |||
| Dinutuximab | Unituxin | mab | chimeric | GD2 ganglioside | Y | neuroblastoma |
| Dinutuximab beta | Qarziba | mab | chimeric | GD2 ganglioside | Y | neuroblastoma |
| Diridavumab | mab | human | Hemagglutinin (influenza) | influenza A | ||
| Divozilimab [29] | Ivlizi | mab | humanized | CD20 | Y (Russia) [60] | multiple sclerosis |
| Domagrozumab [41] | mab | humanized | GDF-8 | Duchenne muscular dystrophy | ||
| Donanemab [35] | Kisunla [61] | mab | humanized | Amyloid beta | Y [61] | Alzheimer's disease |
| Dorlimomab aritox [62] | F(ab')2 | mouse | ||||
| Dostarlimab [63] | Jemperli | mab | humanized | PCDP1 | Y | endometrial cancer |
| Drozitumab | mab | human | DR5 | cancer etc. | ||
| DS-8201 | humanized | HER2 | gastric or gastroesophageal junction adenocarcinoma | |||
| Duligotuzumab [15] | mab | humanized | ERBB3 (HER3) | testicular cancer | ||
| Dupilumab [40] | Dupixent | mab | human | IL-4Rα | Y | atopic dermatitis, asthma, nasal polyps |
| Durvalumab [25] | Imfinzi | mab | human | PD-L1 | Y | cancer |
| Dusigitumab | mab | human | IGF-2 | B-cell malignancies | ||
| Duvortuxizumab [28] | scFv | chimeric/ humanized | CD19, CD3E | cancer | ||
| Ecromeximab [21] | mab | chimeric | GD3 ganglioside | malignant melanoma | ||
| Eculizumab [21] | Soliris | mab | humanized | C5 | Y | paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome |
| Edobacomab | mab | mouse | endotoxin | sepsis caused by Gram-negative bacteria | ||
| Edrecolomab | Panorex | mab | mouse | EpCAM | colorectal carcinoma | |
| Efalizumab [7] | Raptiva | mab | humanized | LFA-1 (CD11a) | psoriasis (blocks T-cell migration) | |
| Efungumab [1] | Mycograb | scFv | human | Hsp90 | invasive Candida infection | |
| Eldelumab [4] | mab | human | CXCL10 (IP-10) | Crohn's disease, ulcerative colitis | ||
| Elezanumab [22] | mab | human | repulsive guidance molecule A (RGMA) | spinal cord injury and multiple sclerosis | ||
| Elgemtumab [25] | mab | human | ERBB3 (HER3) | cancer | ||
| Elotuzumab | Empliciti | mab | humanized | SLAMF7 | Y | multiple myeloma |
| Elsilimomab | mab | mouse | IL-6 | |||
| Emactuzumab [6] | mab | humanized | CSF1R | cancer | ||
| Emapalumab [22] | Gamifant | mab | human | IFN-γ | Y | hemophagocytic lymphohistiocytosis |
| Emibetuzumab | mab | humanized | HGFR | cancer | ||
| Emicizumab [12] | Hemlibra | BsAb | humanized | activated F9, F10 | Y | haemophilia A |
| Enapotamab vedotin [5] | mab | human | AXL | cancer | ||
| Enavatuzumab | mab | humanized | TWEAK receptor | cancer etc. | ||
| Enfortumab vedotin [4] | Padcev | mab | human | nectin-4 | Y [64] | urothelial cancer |
| Enlimomab pegol [65] | mab | mouse | ICAM-1 (CD54) | |||
| Enoblituzumab [22] | mab | humanized | CD276 | cancer | ||
| Enokizumab | mab | humanized | IL-9 | asthma | ||
| Enoticumab [15] | mab | human | DLL4 | |||
| Ensituximab | mab | chimeric | MUC5AC | cancer | ||
| Epcoritamab | Epkinly [27] [51] | BiTE | humanized | CD3, CD20 | Y | diffuse large B-cell lymphoma |
| Epitumomab cituxetan [66] | mab | mouse | episialin | |||
| Epratuzumab | mab | humanized | CD22 | cancer, systemic lupus erythematosus (SLE) | ||
| Eptinezumab [22] | Vyepti | mab | humanized | calcitonin gene-related peptide | Y [67] | migraine |
| Erenumab [22] | Aimovig | mab | human | calcitonin gene-related peptide receptor (CGRP) | Y | migraine |
| Erlizumab [68] | F(ab')2 | humanized | ITGB2 (CD18) | heart attack, stroke, traumatic shock | ||
| Ertumaxomab [31] | Rexomun | 3funct | rat/mouse hybrid | HER2/neu, CD3 | Y | breast cancer etc. |
| Etaracizumab | Abegrin | mab | humanized | integrin αvβ3 | Y | melanoma, prostate cancer, ovarian cancer etc. |
| Etesevimab [29] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | US emergency use authorization (EUA) when used with bamlanivimab [30] | COVID-19 | |
| Etigilimab [5] | mab | humanized | TIGIT | |||
| Etrolizumab [16] | mab | humanized | integrin β7 | inflammatory bowel disease | ||
| Evinacumab | Evkeeza | mab | human | angiopoietin 3 | Y | dyslipidemia |
| Evolocumab [40] | Repatha | mab | human | PCSK9 | Y | hypercholesterolemia |
| Exbivirumab [69] | mab | human | hepatitis B surface antigen | hepatitis B | ||
| Fanolesomab [17] | NeutroSpec | mab | mouse | CD15 | appendicitis (diagnosis) | |
| Faralimomab | mab | mouse | IFN receptor | |||
| Faricimab [5] | Vabysmo | mab | humanized | VEGF-A and Ang-2 | Y | angiogenesis, ocular vascular diseases |
| Farletuzumab | mab | humanized | folate receptor 1 | ovarian cancer | ||
| Fasinumab | mab | human | nerve growth factor (NGF) | acute sciatic pain | ||
| FBTA05 [70] [71] | Lymphomun | 3funct | rat/mouse hybrid | CD20 | chronic lymphocytic leukaemia | |
| Felvizumab | mab | humanized | respiratory syncytial virus | respiratory syncytial virus infection | ||
| Fezakinumab [72] [73] | mab | human | IL-22 | rheumatoid arthritis, psoriasis | ||
| Fibatuzumab [74] | mab | humanized | ephrin receptor A3 | |||
| Ficlatuzumab | mab | humanized | Hepatocyte growth factor (HGF) | cancer etc. | ||
| Figitumumab | mab | human | IGF-1 receptor (CD221) | adrenocortical carcinoma, non-small cell lung carcinoma etc. | ||
| Firivumab [6] | mab | human | Hemagglutinin (influenza) | |||
| Flanvotumab [44] | mab | human | TYRP1 (glycoprotein 75) | melanoma | ||
| Fletikumab | mab | human | IL-20 | rheumatoid arthritis | ||
| Flotetuzumab [5] | di-scFv | humanized | IL-3 receptor | hematological malignancies | ||
| Fontolizumab [21] | HuZAF | mab | humanized | IFN-γ | Crohn's disease etc. | |
| Foralumab [75] | mab | human | CD3E | |||
| Foravirumab [49] | mab | human | rabies virus glycoprotein | rabies (prophylaxis) | ||
| Fremanezumab [22] | Ajovy | mab | humanized | calcitonin gene-related peptide alpha and beta | Y | migraine |
| Fresolimumab [57] | mab | human | TGF-β | idiopathic pulmonary fibrosis, focal segmental glomerulosclerosis, cancer | ||
| Frovocimab [63] | mab | humanized | PCSK9 | hypercholesterolemia | ||
| Frunevetmab [76] | Solensia [77] | mab | veterinary | nerve growth factor (NGF) [77] | Y | pain associated with osteoarthritis in cats [77] |
| Fulranumab | mab | human | nerve growth factor (NGF) | pain | ||
| Futuximab [15] | mab | chimeric | Epidermal growth factor receptor (EGFR) | cancer | ||
| Galcanezumab [41] | Emgality | mab | humanized | calcitonin | Y | migraine |
| Galiximab | mab | chimeric | CD80 | B-cell lymphoma | ||
| Gancotamab | scFv | human | HER2/neu | cancer | ||
| Ganitumab | mab | human | IGF-1 receptor (CD221) | cancer | ||
| Gantenerumab [43] | mab | human | β-amyloid (1-40 and 1-42) | Alzheimer's disease | ||
| Garadacimab [35] | Andembry | mab | human | Coagulation factor XIIa | Y [78] [79] | hereditary angioedema |
| Gatipotuzumab [28] | mab | humanized | MUC1 | cancer | ||
| Gavilimomab [68] | mab | mouse | CD147 (basigin) | graft versus host disease | ||
| Gedivumab [28] | mab | human | Hemagglutinin (influenza) | |||
| Gemtuzumab ozogamicin [22] | Mylotarg | mab | humanized | CD33 | Y | acute myelogenous leukemia |
| Gevokizumab | mab | humanized | IL-1β | diabetes etc. | ||
| Gilvetmab [28] | mab | veterinary | PCDC1 | |||
| Gimsilumab [26] | mab | human | CSF2 | rheumatoid arthritis | ||
| Girentuximab [57] | Rencarex | mab | chimeric | carbonic anhydrase 9 (CA-IX) | clear cell renal cell carcinoma [80] | |
| Glembatumumab vedotin [38] [81] | mab | human | GPNMB | melanoma, breast cancer | ||
| Glofitamab [27] | Columvi [82] | BsAb | humanized | CD20, CD3 | Y [82] | diffuse large B-cell lymphoma [82] |
| Golimumab [69] | Simponi | mab | human | TNF-α | Y | rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis |
| Gomiliximab | mab | chimeric | CD23 (IgE receptor) | allergic asthma | ||
| Gosuranemab | mab | humanized | tau protein | progressive supranuclear palsy | ||
| Guselkumab | Tremfya | mab | human | IL-23 | Y | psoriasis |
| Ianalumab [26] | mab | human | BAFF-R | autoimmune hepatitis | ||
| Ibalizumab [43] | Trogarzo | mab | humanized | CD4 | Y | HIV infection |
| Sintilimab | human | PD-1 | squamous cell non-small cell lung cancer | |||
| Ibritumomab tiuxetan | Zevalin | mab | mouse | CD20 | Y | non-Hodgkin's lymphoma |
| Icrucumab | mab | human | VEGFR-1 | cancer etc. | ||
| Idarucizumab [4] | Praxbind | Fab | humanized | dabigatran | Y | reversal of anticoagulant effects of dabigatran |
| Ifabotuzumab [22] | mab | humanized | EPHA3 | glioblastoma multiforme [83] | ||
| Igovomab | Indimacis-125 | F(ab')2 | mouse | CA-125 | ovarian cancer (diagnosis) | |
| Iladatuzumab vedotin [26] | mab | humanized | CD79B | cancer | ||
| Imalumab [6] | mab | human | macrophage migration inhibitory factor (MIF) | cancer | ||
| Imaprelimab [5] | mab | humanized | melanoma cell adhesion molecule (MCAM) | |||
| Imciromab | Myoscint | mab | mouse | cardiac myosin | Y | cardiac imaging |
| Imdevimab [29] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Yes when used with casirivimab [45] [46] | COVID-19 | |
| Imgatuzumab [15] | mab | humanized | Epidermal growth factor receptor (EGFR) | cancer | ||
| Inclacumab [44] | mab | human | selectin P | cardiovascular disease | ||
| Indatuximab ravtansine [42] | mab | chimeric | SDC1 | cancer | ||
| Indusatumab vedotin [25] | mab | human | GUCY2C | cancer | ||
| Inebilizumab [12] | Uplizna | mab | humanized | CD19 | Y [84] | cancer, systemic sclerosis, multiple sclerosis |
| Infliximab | Remicade | mab | chimeric | TNF-α | Y | rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, Crohn's disease, ulcerative colitis |
| Intetumumab [85] [86] | mab | human | CD51 | solid tumors (prostate cancer, melanoma) | ||
| Inolimomab | mab | mouse | CD25 (α chain of IL-2 receptor) | graft versus host disease | ||
| Inotuzumab ozogamicin [39] | Besponsa | mab | humanized | CD22 | Y | Acute lymphoblastic leukemia (ALL) |
| Ipilimumab [59] | Yervoy | mab | human | CD152 | Y | melanoma |
| Iomab-B | mouse | CD45 | ablation of bone marrow | |||
| Iratumumab [59] | mab | human | CD30 (TNFRSF8) | Hodgkin's lymphoma | ||
| Isatuximab | Sarclisa | mab | chimeric | CD38 | Y | multiple myeloma |
| Iscalimab [5] | mab | human | CD40 | |||
| Istiratumab [26] | mab | human | IGF-1 receptor (CD221) | advanced solid tumors | ||
| Itolizumab [75] | Alzumab | mab | humanized | CD6 | Y | psoriasis |
| Ixekizumab | Taltz | mab | humanized | IL-17A | Y | autoimmune diseases |
| KappaMAB | mab | mouse | Kappa Myeloma Antigen (KMA) | multiple myeloma | ||
| Keliximab | mab | chimeric | CD4 | chronic asthma | ||
| Labetuzumab [7] | CEA-Cide | mab | humanized | Carcinoembryonic antigen (CEA) | colorectal cancer | |
| Lacnotuzumab [28] | mab | humanized | CSF1, macrophage colony stimulating factor (MCSF) | cancer | ||
| Ladiratuzumab vedotin [26] | mab | humanized | LIV-1 | cancer | ||
| Lampalizumab [15] | Fab | humanized | Complement factor D (CFD) | geographic atrophy secondary to age-related macular degeneration | ||
| Lanadelumab [41] | Takhzyro | mab | human | kallikrein | Y | angioedema |
| Landogrozumab [12] | mab | humanized | GDF-8 | muscle wasting disorders | ||
| Laprituximab emtansine [41] | mab | chimeric | epidermal growth factor receptor (EGFR) | |||
| Larcaviximab [28] | mab | chimeric | ebolavirus glycoprotein | Ebola virus | ||
| Lebrikizumab | Ebglyss | mab | humanized | IL-13 | eczema | |
| Lecanemab [87] | Leqembi | mab | humanized | β-amyloid | Y [88] | Alzheimer's disease |
| Lemalesomab [68] | mab | mouse | NCA-90 (granulocyte antigen) | diagnostic agent | ||
| Lendalizumab [41] | mab | humanized | C5 | |||
| Lenvervimab [5] | mab | humanized | hepatitis B surfage antigen | hepatitis B | ||
| Lenzilumab [6] | mab | human | CSF2 | chronic myelomonocytic leukemia and juvenile myelomonocytic leukemia | ||
| Lerdelimumab [17] | mab | human | TGF-β2 | reduction of scarring after glaucoma surgery | ||
| Leronlimab [5] | mab | humanized | CCR5 | breast cancer, HIV | ||
| Lesofavumab [28] | mab | human | Hemagglutinin (influenza) | |||
| Letolizumab [28] | scFv | humanized | tumor necrosis factor related activation protein (TRAP) | inflammatory diseases | ||
| Lexatumumab [1] | mab | human | TRAIL-R2 | cancer | ||
| Libivirumab [69] | mab | human | hepatitis B surface antigen | hepatitis B | ||
| Lifastuzumab vedotin | mab | humanized | phosphate-sodium co-transporter | cancer | ||
| Ligelizumab [15] | mab | humanized | IGHE | severe asthma, chronic spontaneous urticaria | ||
| Loncastuximab tesirine [89] [74] | Zynlonta | mab | chimeric | CD19 | Y | relapsed or refractory large B-cell lymphoma |
| Losatuxizumab vedotin [28] | mab | chimeric/ humanized | epidermal growth factor receptor (EGFR), ERBB1 (HER1) | cancer | ||
| Lilotomab satetraxetan [25] | mab | mouse | CD37 | cancer | ||
| Lintuzumab | mab | humanized | CD33 | cancer | ||
| Linvoseltamab [51] | Lynozyfic [90] [91] | BsAb | human | CD3 and B-cell maturation antigen (BCMA) | Y [90] [91] | multiple myeloma [90] [91] |
| Lirilumab [15] | mab | human | KIR2D | solid and hematological cancers | ||
| Lodelcizumab [40] | mab | humanized | PCSK9 | hypercholesterolemia | ||
| Lokivetmab [25] | Cytopoint [92] | mab | veterinary | Canis lupus familiaris IL31 | Y | clinical signs of atopic dermatitis in dogs [92] |
| Lorvotuzumab mertansine | mab | humanized | CD56 | cancer | ||
| Lucatumumab [13] | mab | human | CD40 | multiple myeloma, non-Hodgkin's lymphoma, Hodgkin's lymphoma | ||
| Lulizumab pegol [6] | mab | humanized | CD28 | autoimmune diseases | ||
| Lumiliximab [9] | mab | chimeric | CD23 (IgE receptor) | chronic lymphocytic leukemia | ||
| Lumretuzumab [6] | mab | humanized | ERBB3 (HER3) | cancer | ||
| Lupartumab [22] | mab | human | ||||
| Lupartumab amadotin [22] | mab | human | LYPD3 | |||
| Lutikizumab [22] | mab | humanized | IL-1α | |||
| Maftivimab | mab | human | part of Atoltivimab/maftivimab/odesivimab for treatment of Zaire ebolavirus (Ebola virus) | |||
| Mapatumumab [31] | mab | human | TRAIL-R1 | cancer | ||
| Margetuximab | Margenza [93] | mab | humanized | HER2 | Y | breast cancer |
| Marstacimab | Hympavzi [94] [95] | mab | human | tissue factor pathway inhibitor (TFPI) | Y | bleeding with hemophilia |
| Maslimomab | mouse | T-cell receptor | ||||
| Mavrilimumab [38] | mab | human | GMCSF receptor α-chain | rheumatoid arthritis | ||
| Matuzumab [24] | mab | humanized | Epidermal growth factor receptor (EGFR) | colorectal, lung and stomach cancer | ||
| Mepolizumab [47] | Nucala | mab | humanized | IL-5 | Y | asthma and white blood cell diseases |
| Metelimumab [24] | mab | human | TGF-β1 | systemic scleroderma | ||
| Milatuzumab [13] | mab | humanized | CD74 | multiple myeloma and other hematological malignancies | ||
| Minretumomab | mab | mouse | TAG-72 | tumor detection (and therapy?) | ||
| Mirikizumab [89] | Omvoh | mab | humanized | IL-23 | Y | ulcerative colitis |
| Mirvetuximab soravtansine [12] | Elahere | mab | chimeric | folate receptor alpha | Y [96] | ovarian cancer |
| Mitumomab | mab | mouse | GD3 ganglioside | small cell lung carcinoma | ||
| Modotuximab | mab | chimeric | EGFR extracellular domain III | cancer | ||
| Mogamulizumab [16] | Poteligeo | mab | humanized | CCR4 | Y | adult T-cell leukemia/lymphoma |
| Monalizumab [12] | mab | humanized | NKG2A | rheumatoid arthritis, gynecologic malignancies, and other cancers | ||
| Morolimumab [97] | mab | human | Rhesus factor | |||
| Mosunetuzumab [89] | Lunsumio | BiTE | humanized | CD3E, MS4A1, CD20 | Y [98] | follicular lymphoma |
| Motavizumab [1] | Numax | mab | humanized | respiratory syncytial virus | respiratory syncytial virus (prevention) | |
| Moxetumomab pasudotox | Lumoxiti | mab | mouse | CD22 | Y | hairy cell leukemia |
| Muromonab-CD3 [99] [100] | Orthoclone OKT3 | mab | mouse | CD3 | prevention of organ transplant rejections | |
| Nacolomab tafenatox | Fab | mouse | C242 antigen | colorectal cancer | ||
| Namilumab [16] | mab | human | CSF2 | |||
| Naptumomab estafenatox [101] | Fab | mouse | 5T4 | non-small cell lung carcinoma, renal cell carcinoma | ||
| Naratuximab emtansine [41] | mab | chimeric | CD37 | |||
| Narnatumab | mab | human | MST1R (aka RON) | cancer | ||
| Natalizumab [97] | Tysabri | mab | humanized | integrin α4 | Y | multiple sclerosis, Crohn's disease |
| Navicixizumab [41] | mab | chimeric/ humanized | DLL4 and VEGFA | cancer | ||
| Navivumab [12] | mab | human | Hemagglutinin (influenza) | |||
| Naxitamab | Danyelza | humanized | c-Met | Y | high-risk neuroblastoma and refractory osteomedullary disease | |
| Nebacumab | mab | human | endotoxin | sepsis | ||
| Necitumumab [102] | Portrazza | mab | human | Epidermal growth factor receptor (EGFR) | Y | non-small cell lung carcinoma |
| Nemolizumab [25] | Nemluvio | mab | humanized | IL-31 receptor A | eczema [103] | |
| NEOD001 | humanized | amyloid | primary systemic amyloidosis | |||
| Nerelimomab | mab | mouse | TNF-α | |||
| Nesvacumab | mab | human | angiopoietin 2 | cancer | ||
| Netakimab [5] | Efleira | mab | humanized | IL-17A | Y (Russia) [104] | plaque psoriasis, psoriatic arthritis, ankylosing spondylitis |
| Nimotuzumab [59] [105] | BioMab-EGFR, Theracim, Theraloc | mab | humanized | epidermal growth factor receptor (EGFR) | Y | squamous cell carcinoma, head and neck cancer, nasopharyngeal cancer, glioma |
| Nipocalimab [87] | Imaavy [106] | mab | human | Neonatal fragment crystallizable receptor | Y [106] | generalized myasthenia gravis [107] [106] |
| Nirsevimab | Beyfortus | mab | human | RSV fusion glycoprotein | respiratory syncytial virus | |
| Nivolumab [6] | Opdivo | mab | human | PD-1 | Y | cancer |
| Nofetumomab merpentan | Verluma | Fab | mouse | cancer (diagnosis) | ||
| Obiltoxaximab | Anthim | mab | chimeric | Bacillus anthracis anthrax | Y | Bacillus anthracis spores |
| Obinutuzumab | Gazyva | mab | humanized | CD20 | Y | chronic lymphatic leukemia |
| Ocaratuzumab | mab | humanized | CD20 | cancer | ||
| Ocrelizumab [108] | Ocrevus | mab | humanized | CD20 | Y | multiple sclerosis |
| Odesivimab | mab | human | part of Atoltivimab/maftivimab/odesivimab for treatment of Zaire ebolavirus (Ebola virus) | |||
| Odulimomab | mab | mouse | LFA-1 (CD11a) | prevention of organ transplant rejections, immunological diseases | ||
| Ofatumumab [31] | Arzerra, Kesimpta [109] | mab | human | CD20 | Y | chronic lymphocytic leukemia, multiple sclerosis [109] |
| Olaratumab | Lartruvo | mab | human | PDGFRA | Y | cancer |
| Oleclumab [28] | mab | human | 5'-nucleotidase | pancreatic and colorectal cancer | ||
| Olendalizumab [28] | mab | humanized | complement C5a | systemic lupus erythematosus, lupus nephritis, acute graft-versus-host disease | ||
| Olokizumab [75] | mab | humanized | IL-6 | rheumatoid arthritis | ||
| Omalizumab [68] | Xolair | mab | humanized | IgE Fc region | Y | allergic asthma, chronic spontaneous urticaria |
| Omburtamab [63] | mab | mouse | CD276 | cancer | ||
| OMS721 | human | MASP-2 | atypical hemolytic uremic syndrome | |||
| Onartuzumab | Fab | humanized | human scatter factor receptor kinase | cancer | ||
| Ontuxizumab | mab | chimeric/ humanized | TEM1 | cancer | ||
| Onvatilimab [5] | mab | human | VISTA (protein) (VSIR) | |||
| Opicinumab | mab | human | LINGO-1 | multiple sclerosis | ||
| Oportuzumab monatox [102] | Vicinium | scFv | humanized | EpCAM | bladder cancer | |
| Oregovomab [17] | OvaRex | mab | mouse | CA-125 | ovarian cancer | |
| Orticumab [15] | mab | human | oxLDL | |||
| Otelixizumab [13] | mab | chimeric/ humanized | CD3 | diabetes mellitus type 1 | ||
| Otilimab | mab | human | GMCSF | osteoarthritis, rheumatoid arthritis | ||
| Otlertuzumab | mab | humanized | CD37 | cancer | ||
| Oxelumab [42] | mab | human | OX-40 | asthma | ||
| Ozanezumab | mab | humanized | NOGO-A | ALS and multiple sclerosis | ||
| Ozoralizumab [42] | mab | humanized | TNF-α | inflammation | ||
| Pagibaximab [31] | mab | chimeric | lipoteichoic acid | sepsis ( Staphylococcus ) | ||
| Palivizumab | Synagis, Abbosynagis | mab | humanized | F protein of respiratory syncytial virus | Y | respiratory syncytial virus (prevention) |
| Pamrevlumab [12] | mab | human | connective tissue growth factor (CTGF) | idiopathic pulmonary fibrosis (IPF), pancreatic cancer | ||
| Panitumumab [69] | Vectibix | mab | human | epidermal growth factor receptor (EGFR) | Y | colorectal cancer |
| Pankomab | mab | humanized | tumor specific glycosylation of MUC1 | ovarian cancer | ||
| Panobacumab [102] | mab | human | Pseudomonas aeruginosa | Pseudomonas aeruginosa infection | ||
| Parsatuzumab [15] | mab | humanized | EGFL7 | cancer | ||
| Pascolizumab [21] | mab | humanized | IL-4 | asthma | ||
| Pasotuxizumab [6] | mab | chimeric/ humanized | folate hydrolase | cancer | ||
| Pateclizumab [42] | mab | humanized | lymphotoxin alpha (LTA) | TNF | ||
| Patritumab [44] | mab | human | ERBB3 (HER3) | cancer | ||
| PDR001 | humanized | PD-1 | melanoma | |||
| Pembrolizumab [56] | Keytruda | mab | humanized | PD-1 | Y [110] | melanoma and other cancers |
| Pemivibart [111] | Pemgarda | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | US emergency use authorization (EUA) [112] | COVID-19 |
| Pemtumomab | Theragyn | mouse | MUC1 | cancer | ||
| Penpulimab [29] | mab | PD-1 | Y [113] | cancer | ||
| Perakizumab [15] | mab | humanized | IL-17A | arthritis | ||
| Pertuzumab | Perjeta | mab | humanized | HER2/neu | Y | cancer |
| Pexelizumab [17] | scFv | humanized | C5 | reduction of side effects of cardiac surgery | ||
| Pidilizumab [40] | mab | humanized | PD-1 | cancer and infectious diseases | ||
| Pinatuzumab vedotin [40] | mab | humanized | CD22 | cancer | ||
| Pintumomab | mab | mouse | adenocarcinoma antigen | adenocarcinoma (imaging) | ||
| Placulumab [15] | mab | human | TNF | pain and inflammatory diseases | ||
| Pozelimab [35] | Veopoz | mab | human | C5 | Y | CHAPLE disease |
| Prezalumab [41] | mab | human | TNF | |||
| Plozalizumab [12] | mab | humanized | CCR2 | diabetic nephropathy and arteriovenous graft patency | ||
| Pogalizumab [41] | mab | humanized | tumor necrosis factor receptor (TNFR) superfamily member 4 | |||
| Polatuzumab vedotin [52] [4] | Polivy | mab | humanized | CD79B | Y | diffuse large B-cell lymphoma |
| Ponezumab | mab | humanized | β-amyloid | Alzheimer's disease | ||
| Porgaviximab [28] | mab | chimeric | Zaire ebolavirus glycoprotein | Ebola virus disease | ||
| Prasinezumab [26] | mab | humanized | Alpha-synuclein | Parkinson's disease | ||
| Prezalizumab [41] | mab | humanized | inducible T-cell co-stimulatory ligand (ICOSL) | |||
| Priliximab | mab | chimeric | CD4 | Crohn's disease, multiple sclerosis | ||
| Pritoxaximab [40] | mab | chimeric | E. coli shiga toxin type-1 | |||
| Pritumumab | mab | human | vimentin | brain cancer | ||
| PRO 140 | humanized | CCR5 | HIV infection | |||
| Quilizumab [44] | mab | humanized | IGHE | asthma | ||
| Racotumomab [102] | Vaxira | mab | mouse | NGNA ganglioside | Y | non-small cell lung cancer |
| Radretumab [16] | mab | human | fibronectin extra domain-B | cancer | ||
| Rafivirumab [49] | mab | human | rabies virus glycoprotein | rabies (prophylaxis) | ||
| Ralpancizumab | mab | humanized | PCSK9 | dyslipidemia | ||
| Ramucirumab | Cyramza | mab | human | VEGFR2 | Y | solid tumors |
| Ranevetmab | mab | veterinary | NGF | osteoarthritis in dogs | ||
| Ranibizumab [9] | Lucentis | Fab | humanized | VEGF-A | Y | macular degeneration (wet form) |
| Raxibacumab [39] | mab | human | anthrax toxin, protective antigen | Y | anthrax (prophylaxis and treatment) | |
| Ravagalimab [5] | mab | humanized | CD40 | Crohn's disease | ||
| Ravulizumab [26] | Ultomiris | mab | humanized | C5 | Y | paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome |
| Refanezumab | mab | humanized | myelin-associated glycoprotein | recovery of motor function after stroke | ||
| Regavirumab | mab | human | cytomegalovirus glycoprotein B | cytomegalovirus infection | ||
| Regdanvimab [29] | Regkirona [114] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Y [114] | COVID-19 |
| Relatlimab | mab | human | LAG3 | melanoma | ||
| Remtolumab [22] | mab | human | IL-17A, TNF | |||
| Reslizumab [7] | Cinqair | mab | humanized | IL-5 | Y | inflammations of the airways, skin and gastrointestinal tract |
| Retifanlimab [27] | Zynyz [115] | mab | humanized | PD-1 | Y | Merkel cell carcinoma |
| Rilotumumab | mab | human | hepatocyte growth factor (HGF) | solid tumors | ||
| Rinucumab | mab | human | PDGFRB | neovascular age-related macular degeneration | ||
| Risankizumab [12] | Skyrizi | mab | humanized | IL-23A | Y | Crohn's disease, psoriasis, psoriatic arthritis, and asthma |
| Rituximab | MabThera, Rituxan | mab | chimeric | CD20 | Y [116] | lymphomas, leukemias, some autoimmune disorders |
| Rivabazumab pegol [12] | mab | humanized | Pseudomonas aeruginosa type III secretion system | |||
| Robatumumab | mab | human | IGF-1 receptor (CD221) | cancer | ||
| Rmab | RabiShield | human | rabies virus G glycoprotein | Y | post-exposure prophylaxis of rabies | |
| Roledumab [75] | mab | human | RHD (gene) (RHD) | Rh disease | ||
| Romilkimab [5] | mab | chimeric/ humanized | IL-13 | |||
| Romosozumab [44] | Evenity | mab | humanized | sclerostin | Y | osteoporosis |
| Rontalizumab [57] | mab | humanized | IFN-α | systemic lupus erythematosus | ||
| Rosmantuzumab [22] | mab | humanized | root plate-specific spondin 3 | cancer | ||
| Rovalpituzumab tesirine [12] | mab | humanized | DLL3 | small cell lung cancer | ||
| Rovelizumab [47] | LeukArrest | mab | humanized | CD11, CD18 | Y | haemorrhagic shock etc. |
| Rozanolixizumab [22] | Rystiggo | mab | chimeric/ humanized | FCGRT | Y [117] | myasthenia gravis |
| Ruplizumab [14] | Antova | mab | humanized | CD154 (CD40L) | Y | rheumatic diseases |
| SA237 | humanized | IL-6 receptor | neuromyelitis optica and neuromyelitis optica spectrum disorders | |||
| Sacituzumab govitecan [22] | Trodelvy | mab | humanized | TROP-2 | Y [118] | triple-negative breast cancer |
| Samalizumab [42] | mab | humanized | CD200 | cancer | ||
| Samrotamab vedotin [5] | mab | chimeric/ humanized | LRRC15 | cancer | ||
| Sarilumab [44] | Kevzara | mab | human | IL-6 | Y | rheumatoid arthritis, ankylosing spondylitis |
| Satralizumab [76] | Enspryng | mab | humanized | IL-6 receptor | Y | neuromyelitis optica |
| Satumomab pendetide | mab | mouse | TAG-72 | cancer (diagnosis) | ||
| Secukinumab | Cosentyx | mab | human | IL-17A | Y | uveitis, rheumatoid arthritis psoriasis |
| Selicrelumab [28] | mab | human | CD40 | |||
| Seribantumab [40] | mab | human | ERBB3 (HER3) | cancer | ||
| Setoxaximab [40] | mab | chimeric | E. coli shiga toxin type-2 | |||
| Setrusumab [26] | mab | human | sclerostin (SOST) | |||
| Sevirumab | human | cytomegalovirus | cytomegalovirus infection | |||
| Sibrotuzumab | mab | humanized | FAP (gene) (FAP) | cancer | ||
| SGN-CD19A | mab | humanized | CD19 | acute lymphoblastic leukemia and B-cell non-Hodgkin lymphoma | ||
| SHP647 | human | mucosal addressin cell adhesion molecule | Crohn's disease | |||
| Sifalimumab [16] | mab | human | IFN-α | systemic lupus erythematosus (SLE), dermatomyositis, polymyositis | ||
| Siltuximab | Sylvant | mab | chimeric | IL-6 | Y | cancer |
| Simtuzumab [15] | mab | humanized | LOXL2 | fibrosis | ||
| Sipavibart [111] | Kavigale [119] [120] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Y [119] | COVID-19 |
| Siplizumab [21] | mab | humanized | CD2 | psoriasis, graft-versus-host disease (prevention) | ||
| Sirtratumab vedotin [26] | mab | human | SLITRK6 | cancer | ||
| Sirukumab | mab | human | IL-6 | rheumatoid arthritis | ||
| Sofituzumab vedotin | mab | humanized | CA-125 | ovarian cancer | ||
| Solanezumab [102] [44] | mab | humanized | β-amyloid | Alzheimer's disease | ||
| Solitomab [44] | BiTE | mouse | EpCAM | gastrointestinal, lung, and other cancers | ||
| Sonepcizumab [121] | humanized | sphingosine-1-phosphate | choroidal and retinal neovascularization | |||
| Sontuzumab [105] | mab | humanized | episialin | |||
| Sotrovimab [29] | Xevudy | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | Y [122] [123] | COVID-19 |
| Spartalizumab [26] | mab | humanized | PD-1 | melanoma | ||
| Spesolimab [54] | Spevigo | mab | humanized | Interleukin 36 receptor (IL1RL2/IL1RAP) | Y | generalized pustular psoriasis (GPP) |
| Stamulumab [108] | mab | human | myostatin | muscular dystrophy | ||
| Sugemalimab [87] | mab | human | PD-1 | non-small-cell lung cancer | ||
| Sulesomab | LeukoScan | Fab' | mouse | NCA-90 (granulocyte antigen) | osteomyelitis (imaging) | |
| Suptavumab [22] | mab | human | RSVFR | medically attended lower respiratory disease | ||
| Sutimlimab [5] | Enjaymo | mab | chimeric/ humanized | complement component 1s (C1s) | Y | cold agglutinin disease |
| Suvizumab [38] | mab | humanized | HIV-1 | viral infections | ||
| Suvratoxumab [28] | mab | human | Staphylococcus aureus alpha toxin | nosocomial pneumonia | ||
| Tabalumab [42] | mab | human | B-cell activating factor (BAFF) | B-cell cancers | ||
| Tacatuzumab tetraxetan | AFP-Cide | mab | humanized | alpha-fetoprotein | cancer | |
| Tadocizumab [105] | Fab | humanized | integrin αIIbβ3 | percutaneous coronary intervention | ||
| Tafasitamab [47] | Monjuvi | mab | humanized (from mouse) | CD19 | Y | relapsed or refractory diffuse large B-cell lymphoma |
| Talacotuzumab [26] | mab | humanized | CD123 | leukemia etc. | ||
| Talizumab [37] | mab | humanized | IgE | allergic reaction | ||
| Talquetamab | Talvey | BsAb | humanized | GPRC5D, CD3 | Y | relapsed or refractory multiple myeloma |
| Tamtuvetmab [41] | Tactress | mab | veterinary | CD52 | ||
| Tanezumab [49] | mab | humanized | nerve growth factor (NGF) | pain | ||
| Taplitumomab paptox [68] | mab | mouse | CD19 | cancer[ citation needed ] | ||
| Tarextumab | mab | human | Notch receptor | cancer | ||
| Tarlatamab [29] | Imdelltra [124] | BsAb | human | DLL3, CD3 [124] | Y [124] | small cell lung cancer [124] |
| Tavolimab | mab | chimeric/ humanized | CD134 | cancer | ||
| Teclistamab | Tecvayli [35] | BsAb | human | B-cell maturation antigen (BCMA), CD3 | Y [125] [126] | relapsed or refractory multiple myeloma |
| Tefibazumab [39] | Aurexis | mab | humanized | clumping factor A | Staphylococcus aureus infection | |
| Telimomab aritox | Fab | mouse | ||||
| Telisotuzumab [22] | mab | humanized | HGFR | cancer | ||
| Telisotuzumab vedotin [22] | mab | humanized | HGFR | cancer | ||
| Tenatumomab [13] | mab | mouse | tenascin C | cancer | ||
| Teneliximab [21] | mab | chimeric | CD40 | autoimmune diseases and prevention of organ transplant rejection | ||
| Teplizumab [43] | Tzield | mab | humanized | CD3 | Y | diabetes mellitus type 1 |
| Tepoditamab [5] | mab | human | dendritic cell-associated lectin 2 | cancer | ||
| Teprotumumab | Tepezza | mab | human | IGF-1 receptor (CD221) | Y | thyroid eye disease |
| Tesidolumab [25] | mab | human | C5 | |||
| Tetulomab | mab | humanized | CD37 | cancer [127] | ||
| Tezepelumab [12] | Tezspire | mab | human | thymic stromal lymphopoietin (TSLP) | Y [128] | asthma |
| TGN1412 | humanized | CD28 | chronic lymphocytic leukemia, rheumatoid arthritis | |||
| Tibulizumab [26] | mab | humanized | B-cell activating factor (BAFF) | autoimmune disorders | ||
| Tildrakizumab | Ilumya | mab | humanized | IL-23 | Y | immunologically mediated inflammatory disorders |
| Tigatuzumab [13] | mab | humanized | TRAIL-R2 | cancer | ||
| Timigutuzumab [28] | mab | humanized | HER2 | cancer | ||
| Timolumab [41] | mab | human | AOC3 | |||
| tiragolumab [26] | mab | human | ||||
| Tiragotumab [26] | mab | human | TIGIT | cancer | ||
| Tislelizumab [26] | mab | humanized | PCDC1, CD279 | non-small cell lung cancer | ||
| Tisotumab vedotin [12] | Tivdak | mab | human | coagulation factor III | Y | relapsed or refractory cervical cancer [129] [130] |
| Tixagevimab [29] | mab | human | spike protein receptor binding domain (RBD) of SARS-CoV-2 | US emergency use authorization (EUA) when used with cilgavimab [48] | COVID-19 | |
| TNX-650 | humanized | IL-13 | Hodgkin's lymphoma | |||
| Tocilizumab [9] | Actemra, RoActemra | mab | humanized | IL-6 receptor | Y [131] | rheumatoid arthritis |
| Tomuzotuximab [28] | mab | humanized | Epidermal growth factor receptor (EGFR), HER1 | cancer | ||
| Toralizumab [21] | mab | humanized | CD154 (CD40L) | rheumatoid arthritis, lupus nephritis etc. | ||
| Tosatoxumab [4] | mab | human | Staphylococcus aureus | |||
| Tositumomab | Bexxar | mouse | CD20 | Y | follicular lymphoma | |
| Tovetumab | mab | human | PDGFRA | cancer | ||
| Tralokinumab [132] | Adtralza, Adbry [133] | mab | human | IL-13 | Y [133] | atopic dermatitis |
| Trastuzumab | Herceptin | mab | humanized | HER2/neu | Y | breast cancer |
| Trastuzumab duocarmazine [22] | Kadcyla | mab | humanized | HER2/neu | Y | breast cancer |
| Trastuzumab emtansine | Kadcyla | mab | humanized | HER2/neu | Y | breast cancer |
| TRBS07 [134] | Ektomab | 3funct | GD2 ganglioside | melanoma | ||
| Tregalizumab [16] | mab | humanized | CD4 | |||
| Tremelimumab [135] | Imjudo | mab | human | CTLA-4 | Y [136] | hepatocellular carcinoma |
| Trevogrumab | mab | human | growth differentiation factor 8 | muscle atrophy due to orthopedic disuse and sarcopenia | ||
| Tucotuzumab celmoleukin [59] [105] | mab | humanized | EpCAM | cancer | ||
| Tuvirumab | human | hepatitis B virus | chronic hepatitis B | |||
| Ublituximab [137] | Briumvi | mab | chimeric | CD20 | Y | multiple sclerosis, chronic lymphocytic leukemia |
| Ulocuplumab [56] | mab | human | CXCR4 (CD184) | hematologic malignancies | ||
| Urelumab [16] | mab | human | 4-1BB (CD137) | cancer etc. | ||
| Urtoxazumab [9] | mab | humanized | Escherichia coli | diarrhoea caused by E. coli | ||
| Ustekinumab [49] | Stelara | mab | human | IL-12, IL-23 | Y | multiple sclerosis, psoriasis, psoriatic arthritis, Crohn's Disease |
| Utomilumab [22] | mab | human | 4-1BB (CD137) | diffuse large B-cell lymphoma | ||
| Vadastuximab talirine [12] | mab | chimeric | CD33 | Acute myeloid leukemia | ||
| Vanalimab [5] | mab | humanized | CD40 | |||
| Vandortuzumab vedotin [25] | mab | humanized | STEAP1 | cancer | ||
| Vantictumab | mab | human | Frizzled receptor | cancer | ||
| Vanucizumab [6] | mab | humanized | angiopoietin 2 | cancer | ||
| Vapaliximab [21] | mab | chimeric | AOC3 (VAP-1) | |||
| Varisacumab [28] | mab | human | VEGF-A | angiogenesis | ||
| Varlilumab [6] | mab | human | CD27 | solid tumors and hematologic malignancies | ||
| Vatelizumab [42] | mab | humanized | ITGA2 (CD49b) | |||
| Vedolizumab [102] | Entyvio | mab | humanized | integrin α4 β7 | Y [138] | Crohn's disease, ulcerative colitis |
| Veltuzumab [13] | mab | humanized | CD20 | non-Hodgkin's lymphoma | ||
| Vepalimomab | mab | mouse | AOC3 (VAP-1) | inflammation | ||
| Vesencumab [16] | mab | human | NRP1 | solid malignancies | ||
| Vilobelimab [87] | Gohibic | mab | chimeric | C5a receptor (C5a) | Y (EU), [139] [140] and US emergency use authorization (EUA) [141] | COVID-19 |
| Visilizumab [68] | Nuvion | mab | humanized | CD3 | Crohn's disease, ulcerative colitis | |
| Vobarilizumab [41] | scFv | humanized | IL-6 receptor | inflammatory autoimmune diseases | ||
| Volociximab [31] | mab | chimeric | integrin α5β1 | solid tumors | ||
| Vonlerolizumab [28] | mab | humanized | CD134 | cancer | ||
| Vopratelimab [5] | mab | humanized | CD278, aka ICOS | |||
| Vorsetuzumab mafodotin | mab | humanized | CD70 | cancer | ||
| Votumumab | HumaSPECT | mab | human | tumor antigen CTAA16.88 | colorectal tumors | |
| Vunakizumab [22] | mab | humanized | IL-17A | |||
| Xentuzumab [41] | mab | humanized | IGF-1, IGF-2 | ? | ||
| XMAB-5574 | humanized | CD19 | diffuse large B-cell lymphoma | |||
| Zalutumumab [31] | mab | human | Epidermal growth factor receptor (EGFR) | squamous cell carcinoma of the head and neck | ||
| Zanidatamab [14] | Ziihera [142] | BsAb | humanized | HER2, HER2 | Y | biliary tract cancer [142] |
| Zanolimumab [39] | mab | human | CD4 | rheumatoid arthritis, psoriasis, T-cell lymphoma | ||
| Zatuximab [15] | mab | chimeric | HER1 | cancer | ||
| Zenocutuzumab [89] | Bizengri [143] | BsAb | humanized | HER2, ERBB3 (HER3) | Y [143] | cancer |
| Ziralimumab [68] | mab | human | CD147 (basigin) | |||
| Zolbetuximab [26] | Vyloy [144] | mab | chimeric | Claudin 18 Isoform 2 | Y | gastric cancer |
| Zolimomab aritox | mab | mouse | CD5 | systemic lupus erythematosus, graft-versus-host disease |
