
Osteoporosis is a systemic skeletal disorder characterized by low bone mass, micro-architectural deterioration of bone tissue leading to more porous bone, and consequent increase in fracture risk. It is the most common reason for a broken bone among the elderly. Bones that commonly break include the vertebrae in the spine, the bones of the forearm, the wrist, and the hip. Until a broken bone occurs there are typically no symptoms. Bones may weaken to such a degree that a break may occur with minor stress or spontaneously. After the broken bone heals, the person may have chronic pain and a decreased ability to carry out normal activities.

Parathyroid hormone (PTH), also called parathormone or parathyrin, is a peptide hormone secreted by the parathyroid glands that regulates the serum calcium concentration through its effects on bone, kidney, and intestine.

Bisphosphonates are a class of drugs that prevent the loss of bone density, used to treat osteoporosis and similar diseases. They are the most commonly prescribed drugs used to treat osteoporosis. They are called bisphosphonates because they have two phosphonate groups. They are thus also called diphosphonates.
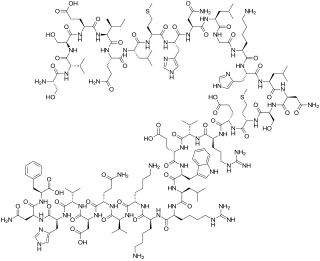
Teriparatide, sold under the brand name Forteo, is a form of parathyroid hormone (PTH) consisting of the first (N-terminus) 34 amino acids, which is the bioactive portion of the hormone. It is an effective anabolic agent used in the treatment of some forms of osteoporosis. Teriparatide is a recombinant human parathyroid hormone analog. It has an identical sequence to the 34 N-terminal amino acids of the 84-amino acid human parathyroid hormone.

Alendronic acid, sold under the brand name Fosamax among others, is a bisphosphonate medication used to treat osteoporosis and Paget's disease of bone. It is taken by mouth. Use is often recommended together with vitamin D, calcium supplementation, and lifestyle changes.

Raloxifene, sold under the brand name Evista among others, is a medication used to prevent and treat osteoporosis in postmenopausal women and those on glucocorticoids. For osteoporosis it is less preferred than bisphosphonates. It is also used to reduce the risk of breast cancer in those at high risk. It is taken by mouth.
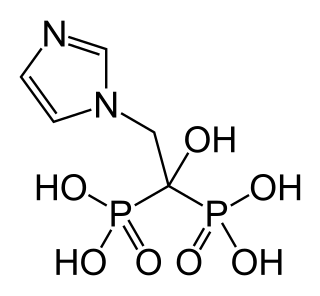
Zoledronic acid, also known as zoledronate and sold under the brand name Zometa among others, by Novartis among others, is a medication used to treat a number of bone diseases. These include osteoporosis, high blood calcium due to cancer, bone breakdown due to cancer, Paget's disease of bone and Duchenne muscular dystrophy (DMD). It is given by injection into a vein.

Osteopenia, known as "low bone mass" or "low bone density", is a condition in which bone mineral density is low. Because their bones are weaker, people with osteopenia may have a higher risk of fractures, and some people may go on to develop osteoporosis. In 2010, 43 million older adults in the US had osteopenia. Unlike osteoporosis, osteopenia does not usually cause symptoms, and losing bone density in itself does not cause pain.
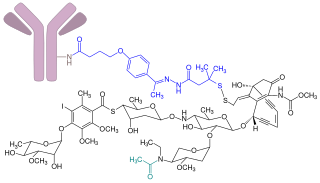
Gemtuzumab ozogamicin, sold under the brand name Mylotarg, is an antibody-drug conjugate that is used to treat acute myeloid leukemia.

Sclerostin is a protein that in humans is encoded by the SOST gene. It is a secreted glycoprotein with a C-terminal cysteine knot-like (CTCK) domain and sequence similarity to the DAN family of bone morphogenetic protein (BMP) antagonists. Sclerostin is produced primarily by the osteocyte but is also expressed in other tissues, and has anti-anabolic effects on bone formation.

Ibandronic acid is a bisphosphonate medication used in the prevention and treatment of osteoporosis and metastasis-associated skeletal fractures in people with cancer. It may also be used to treat hypercalcemia. It is typically formulated as its sodium salt ibandronate sodium.

Denosumab, sold under the brand names Prolia and Xgeva among others, is a human monoclonal antibody used for the treatment of osteoporosis, treatment-induced bone loss, metastases to bone, and giant cell tumor of bone.

Strontium ranelate, a strontium(II) salt of ranelic acid, is a medication for osteoporosis marketed as Protelos or Protos by Servier. Studies indicate it can also slow the course of osteoarthritis of the knee. The drug is unusual in that it both increases deposition of new bone by osteoblasts and reduces the resorption of bone by osteoclasts. It is therefore promoted as a "dual action bone agent" (DABA).

Lasofoxifene, sold under the brand name Fablyn, is a nonsteroidal selective estrogen receptor modulator (SERM) which is marketed by Pfizer in Lithuania and Portugal for the prevention and treatment of osteoporosis and for the treatment of vaginal atrophy, and the result of an exclusive research collaboration with Ligand Pharmaceuticals (LGND). It also appears to have had a statistically significant effect of reducing breast cancer in women according to a study published in The Journal of the National Cancer Institute.
Senile osteoporosis has been recently recognized as a geriatric syndrome with a particular pathophysiology. There are different classification of osteoporosis: primary, in which bone loss is a result of aging and secondary, in which bone loss occurs from various clinical and lifestyle factors. Primary, or involuntary osteoporosis, can further be classified into Type I or Type II. Type I refers to postmenopausal osteoporosis and is caused by the deficiency of estrogen. While senile osteoporosis is categorized as an involuntary, Type II, and primary osteoporosis, which affects both men and women over the age of 70 years. It is accompanied by vitamin D deficiency, body's failure to absorb calcium, and increased parathyroid hormone.
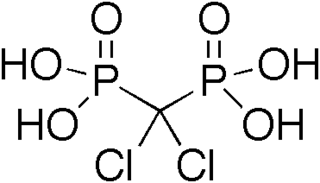
Clodronic acid (INN) or clodronate disodium (Na2CH2Cl2O6P2) (USAN) is a first generation (non-nitrogenous) bisphosphonate. It is an anti-osteoporotic drug approved for the prevention and treatment of osteoporosis in post-menopausal women and men to reduce vertebral fractures, hyperparathyroidism, hypercalcemia in malignancy, multiple myeloma and fracture related pain because of its anti-inflammatory effects shown as a reduction in inflammatory markers like IL-1β, IL-6, and TNF-α.
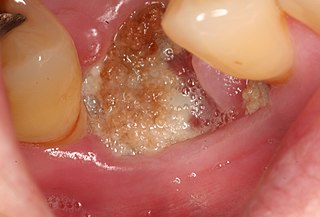
Medication-related osteonecrosis of the jaw is progressive death of the jawbone in a person exposed to a medication known to increase the risk of disease, in the absence of a previous radiation treatment. It may lead to surgical complication in the form of impaired wound healing following oral and maxillofacial surgery, periodontal surgery, or endodontic therapy.
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause. Effects of menopause can include symptoms such as hot flashes, accelerated skin aging, vaginal dryness, decreased muscle mass, and complications such as osteoporosis, sexual dysfunction, and vaginal atrophy. They are mostly caused by low levels of female sex hormones that occur during menopause.
Abaloparatide, sold under the brand name Tymlos among others, is a parathyroid hormone-related protein (PTHrP) analog medication used to treat osteoporosis. It is an anabolic agent.
Recombinant human parathyroid hormone, sold under the brand name Preotact among others, is an artificially manufactured form of the parathyroid hormone used to treat hypoparathyroidism. Recombinant human parathyroid hormone is used in the treatment of osteoporosis in postmenopausal women at high risk of osteoporotic fractures. A significant reduction in the incidence of vertebral fractures has been demonstrated. It is used in combination with calcium and vitamin D supplements.















