
An anticoagulant, commonly known as a blood thinner, is a chemical substance that prevents or reduces the coagulation of blood, prolonging the clotting time. Some occur naturally in blood-eating animals, such as leeches and mosquitoes, which help keep the bite area unclotted long enough for the animal to obtain blood.

Venous thrombosis is the blockage of a vein caused by a thrombus. A common form of venous thrombosis is deep vein thrombosis (DVT), when a blood clot forms in the deep veins. If a thrombus breaks off (embolizes) and flows to the lungs to lodge there, it becomes a pulmonary embolism (PE), a blood clot in the lungs. The conditions of DVT only, DVT with PE, and PE only, are all captured by the term venous thromboembolism (VTE).

Warfarin is an anticoagulant used as a medication under several brand names including Coumadin. While the drug is described as a "blood thinner", it does not reduce viscosity but rather inhibits coagulation. Accordingly, it is commonly used to prevent blood clots in the circulatory system such as deep vein thrombosis and pulmonary embolism, and to protect against stroke in people who have atrial fibrillation, valvular heart disease, or artificial heart valves. Less commonly, it is used following ST-segment elevation myocardial infarction (STEMI) and orthopedic surgery. It is usually taken by mouth, but may also be administered intravenously.
Low-molecular-weight heparin (LMWH) is a class of anticoagulant medications. They are used in the prevention of blood clots and, in the treatment of venous thromboembolism, and the treatment of myocardial infarction.

The prothrombin time (PT) – along with its derived measures of prothrombin ratio (PR) and international normalized ratio (INR) – is an assay for evaluating the extrinsic pathway and common pathway of coagulation. This blood test is also called protime INR and PT/INR. They are used to determine the clotting tendency of blood, in such things as the measure of warfarin dosage, liver damage, and vitamin K status. PT measures the following coagulation factors: I (fibrinogen), II (prothrombin), V (proaccelerin), VII (proconvertin), and X.
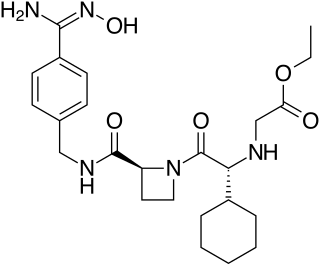
Ximelagatran is an anticoagulant that has been investigated extensively as a replacement for warfarin that would overcome the problematic dietary, drug interaction, and monitoring issues associated with warfarin therapy. In 2006, its manufacturer AstraZeneca announced that it would withdraw pending applications for marketing approval after reports of hepatotoxicity during trials, and discontinue its distribution in countries where the drug had been approved.
Protamine sulfate is a medication that is used to reverse the effects of heparin. It is specifically used in heparin overdose, in low molecular weight heparin overdose, and to reverse the effects of heparin during delivery and heart surgery. It is given by injection into a vein. The onset of effects is typically within five minutes.

Enoxaparin sodium, sold under the brand name Lovenox among others, is an anticoagulant medication. It is used to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE) including during pregnancy and following certain types of surgery. It is also used in those with acute coronary syndrome (ACS) and heart attacks. It is given by injection just under the skin or into a vein. It is also used during hemodialysis.
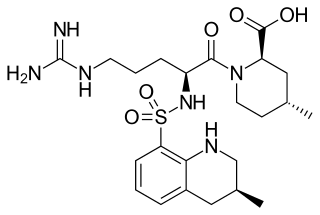
Argatroban is an anticoagulant that is a small molecule direct thrombin inhibitor. In 2000, argatroban was licensed by the Food and Drug Administration (FDA) for prophylaxis or treatment of thrombosis in patients with heparin-induced thrombocytopenia (HIT). In 2002, it was approved for use during percutaneous coronary interventions in patients who have HIT or are at risk for developing it. In 2012, it was approved by the MHRA in the UK for anticoagulation in patients with heparin-induced thrombocytopenia Type II (HIT) who require parenteral antithrombotic therapy.

Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication used to treat and prevent blood clots. Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery. It is taken by mouth.

Dabigatran, sold under the brand name Pradaxa among others, is an anticoagulant used to treat and prevent blood clots and to prevent stroke in people with atrial fibrillation. Specifically it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests. In a meta analysis of 7 different studies, there was no benefit of dabigatran over warfarin in preventing ischemic stroke; however, dabigatran were associated with a lower hazard for intracranial bleeding compared with warfarin, but also had a higher risk of gastrointestinal bleeding relative to warfarin. It is taken by mouth.
Prothrombin complex concentrate (PCC), also known as factor IX complex, sold under the brand name Kcentra among others, is a combination medication made up of blood clotting factors II, IX, and X. Some versions also contain factor VII. It is used to treat and prevent bleeding in hemophilia B if pure factor IX is not available. It may also be used for reversal of warfarin therapy. It is given by slow injection into a vein.
Direct thrombin inhibitors (DTIs) are a class of medication that act as anticoagulants by directly inhibiting the enzyme thrombin. Some are in clinical use, while others are undergoing clinical development. Several members of the class are expected to replace heparin and warfarin in various clinical scenarios.
Hypercoagulability in pregnancy is the propensity of pregnant women to develop thrombosis. Pregnancy itself is a factor of hypercoagulability, as a physiologically adaptive mechanism to prevent post partum bleeding. However, when combined with an additional underlying hypercoagulable states, the risk of thrombosis or embolism may become substantial.

Vitamin K antagonists (VKA) are a group of substances that reduce blood clotting by reducing the action of vitamin K. The term "vitamin K antagonist" is technically a misnomer, as the drugs do not directly antagonize the action of vitamin K in the pharmacological sense, but rather the recycling of vitamin K. Vitamin K antagonists (VKAs) have been the mainstay of anticoagulation therapy for more than 50 years.
Jonathan L. Halperin is an American cardiologist and the author of Bypass (ISBN 0-89586-509-2), among the most comprehensive works on the subject of coronary artery bypass surgery. In addition, he is the Robert and Harriet Heilbrunn Professor of Medicine at The Mount Sinai School of Medicine as well as Director of Clinical Cardiology in the Zena and Michael A. Wierner Cardiovascular Institute at The Mount Sinai Medical Center, both in New York City. Halperin was the principal cardiologist responsible for both the design and execution of the multi-center Stroke Prevention in Atrial Fibrillation (SPAF) clinical trials, funded by the National Institutes of Health, which helped develop antithrombotic strategies to prevent stroke, and he subsequently directed the SPORTIF clinical trials, which evaluated the first oral direct thrombin inhibitor for prevention of stroke in patients with atrial fibrillation.

Apixaban, sold under the brand name Eliquis, is an anticoagulant medication used to treat and prevent blood clots and to prevent stroke in people with nonvalvular atrial fibrillation through directly inhibiting factor Xa. Specifically, it is used to prevent blood clots following hip or knee replacement and in those with a history of prior clots. It is used as an alternative to warfarin and does not require monitoring by blood tests or dietary restrictions. It is taken by mouth.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.
Andexanet alfa, sold under the brand name Andexxa among others, is an antidote for the medications rivaroxaban and apixaban, when reversal of anticoagulation is needed due to uncontrolled bleeding. It has not been found to be useful for other factor Xa inhibitors. It is given by injection into a vein.

Ciraparantag (aripazine) is a drug under investigation as an antidote for a number of anticoagulant drugs, including factor Xa inhibitors, dabigatran, and heparins.











