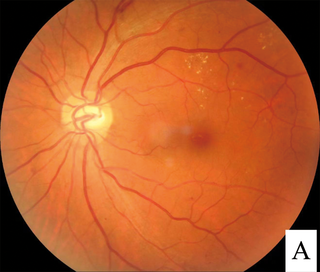
Diabetic retinopathy, is a medical condition in which damage occurs to the retina due to diabetes mellitus. It is a leading cause of blindness in developed countries.
The National Eye Institute (NEI) is part of the U.S. National Institutes of Health (NIH), an agency of the U.S. Department of Health and Human Services. The mission of NEI is "to eliminate vision loss and improve quality of life through vision research." NEI consists of two major branches for research: an extramural branch that funds studies outside NIH and an intramural branch that funds research on the NIH campus in Bethesda, Maryland. Most of the NEI budget funds extramural research.

Macular edema occurs when fluid and protein deposits collect on or under the macula of the eye and causes it to thicken and swell (edema). The swelling may distort a person's central vision, because the macula holds tightly packed cones that provide sharp, clear, central vision to enable a person to see detail, form, and color that is directly in the centre of the field of view.

Macular degeneration, also known as age-related macular degeneration, is a medical condition which may result in blurred or no vision in the center of the visual field. Early on there are often no symptoms. Over time, however, some people experience a gradual worsening of vision that may affect one or both eyes. While it does not result in complete blindness, loss of central vision can make it hard to recognize faces, drive, read, or perform other activities of daily life. Visual hallucinations may also occur.
Bevacizumab, sold under the brand name Avastin among others, is a medication used to treat a number of types of cancers and a specific eye disease. For cancer, it is given by slow injection into a vein (intravenous) and used for colon cancer, lung cancer, glioblastoma, and renal-cell carcinoma. In many of these diseases it is used as a first-line therapy. For age-related macular degeneration it is given by injection into the eye (intravitreal).
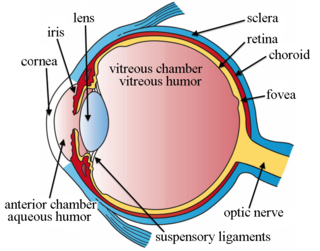
Intravitreal is a route of administration of a drug, or other substance, in which the substance is delivered into the vitreous humor of the eye. "Intravitreal" literally means "inside an eye". Intravitreal injections were first introduced in 1911 when Ohm gave an injection of air into the vitreous humor to repair a detached retina. In the mid-1940s, intravitreal injections became a standard way to administer drugs to treat endophthalmitis and cytomegalovirus retinitis.
Ranibizumab, sold under the brand name Lucentis among others, is a monoclonal antibody fragment (Fab) created from the same parent mouse antibody as bevacizumab. It is an anti-angiogenic that is approved to treat the "wet" type of age-related macular degeneration, diabetic retinopathy, and macular edema due to branch retinal vein occlusion or central retinal vein occlusion.

Pegaptanib sodium injection is an anti-angiogenic medicine for the treatment of neovascular (wet) age-related macular degeneration (AMD). It was discovered by NeXstar Pharmaceuticals and licensed in 2000 to EyeTech Pharmaceuticals, now OSI Pharmaceuticals, for late stage development and marketing in the United States. Gilead Sciences continues to receive royalties from the drugs licensing. Outside the US pegaptanib is marketed by Pfizer. Approval was granted by the U.S. Food and Drug Administration (FDA) in December 2004.
Aflibercept, sold under the brand names Eylea among others, is a medication used to treat wet macular degeneration and metastatic colorectal cancer. It was developed by Regeneron Pharmaceuticals and is approved in the United States and the European Union.

Macular telangiectasia is a condition of the retina, the light-sensing tissue at the back of the eye that causes gradual deterioration of central vision, interfering with tasks such as reading and driving.
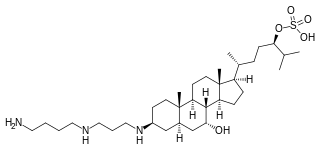
Squalamine is a steroid-polyamine conjugate compound with broad spectrum antimicrobial activity and anti-angiogenic activity. It was studied as a potential cancer drug and as a potential treatment for wet macular degeneration but as of 2018 had not succeeded in Phase III trials for any use.

Laser coagulation or laser photocoagulation surgery is used to treat a number of eye diseases and has become widely used in recent decades. During the procedure, a laser is used to finely cauterize ocular blood vessels to attempt to bring about various therapeutic benefits.
DARPins are genetically engineered antibody mimetic proteins typically exhibiting highly specific and high-affinity target protein binding. They are derived from natural ankyrin repeat proteins, one of the most common classes of binding proteins in nature, which are responsible for diverse functions such as cell signaling, regulation and structural integrity of the cell. DARPins consist of at least three, repeat motifs or modules, of which the most N- and the most C-terminal modules are referred to as "caps", since they shield the hydrophobic core of the protein. The number of internal modules is indicated as number while the caps are indicated with "N" or "C", respectively. The molecular mass of e.g. 14 or 18 kDa (kilodaltons) for four- (N2C) or five- (N3C) repeat DARPins is rather small for a biologic.
Nesvacumab is an experimental monoclonal antibody originally designed for the treatment of cancer. It targets the protein angiopoietin 2. As of May 2017, it is in Phase II clinical trials for the treatment of diabetic macular edema.
Joan Whitten Miller is a Canadian-American ophthalmologist and scientist who has made notable contributions to the treatment and understanding of eye disorders. She is credited for developing photodynamic therapy (PDT) with verteporfin (Visudyne), the first pharmacologic therapy for retinal disease. She also co-discovered the role of vascular endothelial growth factor (VEGF) in eye disease and demonstrated the therapeutic potential of VEGF inhibitors, forming the scientific basis of anti-VEGF therapy for age-related macular degeneration (AMD), diabetic retinopathy, and related conditions.
Anti–vascular endothelial growth factor therapy, also known as anti-VEGF therapy or medication, is the use of medications that block vascular endothelial growth factor. This is done in the treatment of certain cancers and in age-related macular degeneration. They can involve monoclonal antibodies such as bevacizumab, antibody derivatives such as ranibizumab (Lucentis), or orally-available small molecules that inhibit the tyrosine kinases stimulated by VEGF: sunitinib, sorafenib, axitinib, and pazopanib.

Emixustat is a small molecule notable for its establishment of a new class of compounds known as visual cycle modulators (VCMs). Formulated as the hydrochloride salt, emixustat hydrochloride, it is the first synthetic medicinal compound shown to affect retinal disease processes when taken by mouth. Emixustat was invented by the British-American chemist, Ian L. Scott, and is currently in Phase 3 trials for dry, age-related macular degeneration (AMD).
Brolucizumab sold under trade name Beovu among others, is a humanized single-chain antibody fragment for the treatment of neovascular (wet) age-related macular degeneration (AMD).

Intravitreal injection is the method of administration of drugs into the eye by injection with a fine needle. The medication will be directly applied into the vitreous humor. It is used to treat various eye diseases, such as age-related macular degeneration (AMD), diabetic retinopathy, and infections inside the eye such as endophthalmitis. As compared to topical administration, this method is beneficial for a more localized delivery of medications to the targeted site, as the needle can directly pass through the anatomical eye barrier and dynamic barrier. It could also minimize adverse drug effects on other body tissues via the systemic circulation, which could be a possible risk for intravenous injection of medications. Although there are risks of infections or other complications, with suitable precautions throughout the injection process, chances for these complications could be lowered.
Conbercept, sold under the commercial name Lumitin, is a novel vascular endothelial growth factor (VEGF) inhibitor used to treat neovascular age-related macular degeneration (AMD) and diabetic macular edema (DME). The anti-VEGF was approved for the treatment of neovascular AMD by the China State FDA (CFDA) in December 2013. As of December 2020, conbercept is undergoing phase III clinical trials through the U.S. Food and Drug Administration’s PANDA-1 and PANDA-2 development programs.









