
In most vertebrates, myelin is a lipid-rich material that surrounds nerve cell axons to insulate them and increase the rate at which electrical impulses are passed along the axon. The myelinated axon can be likened to an electrical wire with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Rather, myelin sheaths the nerve in segments: in general, each axon is encased with multiple long myelinated sections with short gaps in between called nodes of Ranvier.

The retina is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then processes that image within the retina and sends nerve impulses along the optic nerve to the visual cortex to create visual perception. The retina serves a function which is in many ways analogous to that of the film or image sensor in a camera.

In neuroanatomy, the optic chiasm, or optic chiasma, is the part of the brain where the optic nerves cross. It is located at the bottom of the brain immediately inferior to the hypothalamus. The optic chiasm is found in all vertebrates, although in cyclostomes, it is located within the brain.

In neuroanatomy, the optic nerve, also known as the second cranial nerve, cranial nerve II, or simply CN II, is a paired cranial nerve that transmits visual information from the retina to the brain. In humans, the optic nerve is derived from optic stalks during the seventh week of development and is composed of retinal ganglion cell axons and glial cells; it extends from the optic disc to the optic chiasma and continues as the optic tract to the lateral geniculate nucleus, pretectal nuclei, and superior colliculus.

A photoreceptor cell is a specialized type of neuroepithelial cell found in the retina that is capable of visual phototransduction. The great biological importance of photoreceptors is that they convert light into signals that can stimulate biological processes. To be more specific, photoreceptor proteins in the cell absorb photons, triggering a change in the cell's membrane potential.

A retinal ganglion cell (RGC) is a type of neuron located near the inner surface of the retina of the eye. It receives visual information from photoreceptors via two intermediate neuron types: bipolar cells and retina amacrine cells. Retina amacrine cells, particularly narrow field cells, are important for creating functional subunits within the ganglion cell layer and making it so that ganglion cells can observe a small dot moving a small distance. Retinal ganglion cells collectively transmit image-forming and non-image forming visual information from the retina in the form of action potential to several regions in the thalamus, hypothalamus, and mesencephalon, or midbrain.

The optic disc or optic nerve head is the point of exit for ganglion cell axons leaving the eye. Because there are no rods or cones overlying the optic disc, it corresponds to a small blind spot in each eye.

Peripherin is a type III intermediate filament protein expressed mainly in neurons of the peripheral nervous system. It is also found in neurons of the central nervous system that have projections toward peripheral structures, such as spinal motor neurons. Its size, structure, and sequence/location of protein motifs is similar to other type III intermediate filament proteins such as desmin, vimentin and glial fibrillary acidic protein. Like these proteins, peripherin can self-assemble to form homopolymeric filamentous networks, but it can also heteropolymerize with neurofilaments in several neuronal types. This protein in humans is encoded by the PRPH gene. Peripherin is thought to play a role in neurite elongation during development and axonal regeneration after injury, but its exact function is unknown. It is also associated with some of the major neuropathologies that characterize amyotropic lateral sclerosis (ALS), but despite extensive research into how neurofilaments and peripherin contribute to ALS, their role in this disease is still unidentified.
In cellular neuroscience, an axotomy is the cutting or otherwise severing of an axon. This type of denervation is often used in experimental studies on neuronal physiology and neuronal death or survival as a method to better understand nervous system diseases.

Retinotopy is the mapping of visual input from the retina to neurons, particularly those neurons within the visual stream. For clarity, 'retinotopy' can be replaced with 'retinal mapping', and 'retinotopic' with 'retinally mapped'.
Gliosis is a nonspecific reactive change of glial cells in response to damage to the central nervous system (CNS). In most cases, gliosis involves the proliferation or hypertrophy of several different types of glial cells, including astrocytes, microglia, and oligodendrocytes. In its most extreme form, the proliferation associated with gliosis leads to the formation of a glial scar.
Neurotrophic factors (NTFs) are a family of biomolecules – nearly all of which are peptides or small proteins – that support the growth, survival, and differentiation of both developing and mature neurons. Most NTFs exert their trophic effects on neurons by signaling through tyrosine kinases, usually a receptor tyrosine kinase. In the mature nervous system, they promote neuronal survival, induce synaptic plasticity, and modulate the formation of long-term memories. Neurotrophic factors also promote the initial growth and development of neurons in the central nervous system and peripheral nervous system, and they are capable of regrowing damaged neurons in test tubes and animal models. Some neurotrophic factors are also released by the target tissue in order to guide the growth of developing axons. Most neurotrophic factors belong to one of three families: (1) neurotrophins, (2) glial cell-line derived neurotrophic factor family ligands (GFLs), and (3) neuropoietic cytokines. Each family has its own distinct cell signaling mechanisms, although the cellular responses elicited often do overlap.
Optic neuropathy is damage to the optic nerve from any cause. The optic nerve is a bundle of millions of fibers in the retina that sends visual signals to the brain. [1].
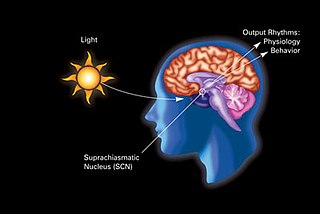
In neuroanatomy, the retinohypothalamic tract (RHT) is a photic neural input pathway involved in the circadian rhythms of mammals. The origin of the retinohypothalamic tract is the intrinsically photosensitive retinal ganglion cells (ipRGC), which contain the photopigment melanopsin. The axons of the ipRGCs belonging to the retinohypothalamic tract project directly, monosynaptically, to the suprachiasmatic nuclei (SCN) via the optic nerve and the optic chiasm. The suprachiasmatic nuclei receive and interpret information on environmental light, dark and day length, important in the entrainment of the "body clock". They can coordinate peripheral "clocks" and direct the pineal gland to secrete the hormone melatonin.

Nerve injury is an injury to nervous tissue. There is no single classification system that can describe all the many variations of nerve injuries. In 1941, Seddon introduced a classification of nerve injuries based on three main types of nerve fiber injury and whether there is continuity of the nerve. Usually, however, peripheral nerve injuries are classified in five stages, based on the extent of damage to both the nerve and the surrounding connective tissue, since supporting glial cells may be involved.

S100 calcium-binding protein A9 (S100A9) also known as migration inhibitory factor-related protein 14 (MRP14) or calgranulin B is a protein that in humans is encoded by the S100A9 gene.

Leucine rich repeat and Immunoglobin-like domain-containing protein 1 also known as LINGO-1 is a protein which is encoded by the LINGO1 gene in humans. It belongs to the family of leucine-rich repeat proteins which are known for playing key roles in the biology of the central nervous system. LINGO-1 is a functional component of the Nogo receptor also known as the reticulon 4 receptor.

A midget cell is one type of retinal ganglion cell (RGC). Midget cells originate in the ganglion cell layer of the retina, and project to the parvocellular layers of the lateral geniculate nucleus (LGN). The axons of midget cells travel through the optic nerve and optic tract, ultimately synapsing with parvocellular cells in the LGN. These cells are known as midget retinal ganglion cells due to the small sizes of their dendritic trees and cell bodies. About 80% of RGCs are midget cells. They receive inputs from relatively few rods and cones. In many cases, they are connected to midget bipolar cells, which are linked to one cone each.
Erythropoietin in neuroprotection is the use of the glycoprotein erythropoietin (Epo) for neuroprotection. Epo controls erythropoiesis, or red blood cell production.
Immune system contribution to regeneration of tissues generally involves specific cellular components, transcription of a wide variety of genes, morphogenesis, epithelia renewal and proliferation of damaged cell types. However, current knowledge reveals more and more studies about immune system influence that cannot be omitted. As the immune system exhibits inhibitory or inflammatory functions during regeneration, the therapies are focused on either stopping these processes or control the immune cells setting in a regenerative way, suggesting that interplay between damaged tissue and immune system response must be well-balanced. Recent studies provide evidence that immune components are required not only after body injury but also in homeostasis or senescent cells replacement.













