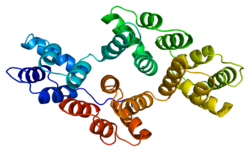Protein S is a vitamin K-dependent plasma glycoprotein synthesized in the liver. In the circulation, Protein S exists in two forms: a free form and a complex form bound to complement protein C4b-binding protein (C4BP). In humans, protein S is encoded by the PROS1 gene. Protein S plays a role in coagulation.
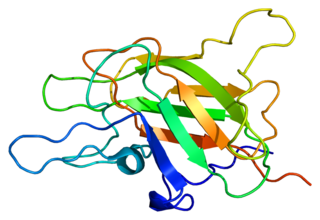
Factor V is a protein of the coagulation system, rarely referred to as proaccelerin or labile factor. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor. Deficiency leads to predisposition for hemorrhage, while some mutations predispose for thrombosis.
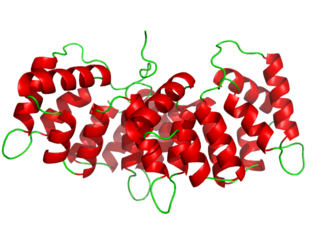
Annexin is a common name for a group of cellular proteins. They are mostly found in eukaryotic organisms.

Dilute Russell's viper venom time (dRVVT) is a laboratory test often used for detection of lupus anticoagulant (LA).
The prothrombinase enzyme complex consists of factor Xa (a serine protease) and factor Va (a protein cofactor). The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (factor II), an inactive zymogen, to thrombin (factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg271 and the other after Arg320. Although it has been shown that factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 105-fold faster than can factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.
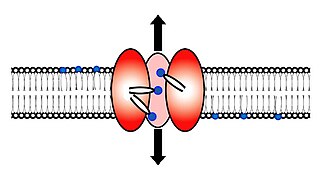
Scramblase is a protein responsible for the translocation of phospholipids between the two monolayers of a lipid bilayer of a cell membrane. In humans, phospholipid scramblases (PLSCRs) constitute a family of five homologous proteins that are named as hPLSCR1–hPLSCR5. Scramblases are members of the general family of transmembrane lipid transporters known as flippases. Scramblases are distinct from flippases and floppases. Scramblases, flippases, and floppases are three different types of enzymatic groups of phospholipid transportation enzymes. The inner-leaflet, facing the inside of the cell, contains negatively charged amino-phospholipids and phosphatidylethanolamine. The outer-leaflet, facing the outside environment, contains phosphatidylcholine and sphingomyelin. Scramblase is an enzyme, present in the cell membrane, that can transport (scramble) the negatively charged phospholipids from the inner-leaflet to the outer-leaflet, and vice versa.
In molecular biology, an annexin A5 affinity assay is a test to quantify the number of cells undergoing apoptosis. The assay uses the protein annexin A5 to tag apoptotic and dead cells, and the numbers are then counted using either flow cytometry or a fluorescence microscope.

Phosphatidylethanolamine (PE) is a class of phospholipids found in biological membranes. They are synthesized by the addition of cytidine diphosphate-ethanolamine to diglycerides, releasing cytidine monophosphate. S-Adenosyl methionine can subsequently methylate the amine of phosphatidylethanolamines to yield phosphatidylcholines.

Annexin A1, also known as lipocortin I, is a protein that is encoded by the ANXA1 gene in humans.

Annexin A2 also known as annexin II is a protein that in humans is encoded by the ANXA2 gene.

β2-glycoprotein 1, also known as beta-2 glycoprotein 1 and Apolipoprotein H (Apo-H), is a 38 kDa multifunctional plasma protein that in humans is encoded by the APOH gene. One of its functions is to bind cardiolipin. When bound, the structure of cardiolipin and β2-GP1 both undergo large changes in structure. Within the structure of Apo-H is a stretch of positively charged amino acids, Lys-Asn-Lys-Glu-Lys-Lys, are involved in phospholipid binding.
In autoimmune disease, anti-apolipoprotein H (AAHA) antibodies, also called anti-β2 glycoprotein I antibodies, comprise a subset of anti-cardiolipin antibodies and lupus anticoagulant. These antibodies are involved in sclerosis and are strongly associated with thrombotic forms of lupus. As a result, AAHA are strongly implicated in autoimmune deep vein thrombosis.

Annexin A6 is a protein that in humans is encoded by the ANXA6 gene.

Discoidin domain is major protein domain of many blood coagulation factors.

S100 calcium-binding protein A10 (S100A10), also known as p11, is a protein that is encoded by the S100A10 gene in humans and the S100a10 gene in other species. S100A10 is a member of the S100 family of proteins containing two EF-hand calcium-binding motifs. S100 proteins are localized in the cytoplasm and/or nucleus of a wide range of cells. They regulate a number of cellular processes such as cell cycle progression and differentiation. The S100 protein is implicated in exocytosis and endocytosis by reorganization of F-actin.

Annexin A4 is a protein that in humans is encoded by the ANXA4 gene.

Annexin A7 is a protein that in humans is encoded by the ANXA7 gene.

Annexin A11 is a protein that in humans is encoded by the ANXA11 gene.

Annexin A3 is a protein that in humans is encoded by the ANXA3 gene.

Annexin A8-like protein 2 is a protein that in humans is encoded by the ANXA8L2 gene.