
Nidogen-1 (NID-1), formerly known as entactin, is a protein that in humans is encoded by the NID1 gene. Both nidogen-1 and nidogen-2 are essential components of the basement membrane alongside other components such as type IV collagen, proteoglycans, laminin and fibronectin.

Laminins are a family of glycoproteins of the extracellular matrix of all animals. They are major components of the basal lamina, the protein network foundation for most cells and organs. The laminins are an important and biologically active part of the basal lamina, influencing cell differentiation, migration, and adhesion.

Endostatin is a naturally occurring, 20-kDa C-terminal fragment derived from type XVIII collagen. It is reported to serve as an anti-angiogenic agent, similar to angiostatin and thrombospondin.
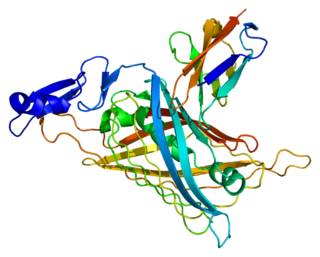
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the HSPG2 gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins.

Fibulin (FY-beau-lin) is the prototypic member of a multigene family, currently with seven members. Fibulin-1 is a calcium-binding glycoprotein. In vertebrates, fibulin-1 is found in blood and extracellular matrices. In the extracellular matrix, fibulin-1 associates with basement membranes and elastic fibers. The association with these matrix structures is mediated by its ability to interact with numerous extracellular matrix constituents including fibronectin, proteoglycans, laminins and tropoelastin. In blood, fibulin-1 binds to fibrinogen and incorporates into clots.

FBLN1 is the gene encoding fibulin-1, an extracellular matrix and plasma protein.

Tenascin C (TN-C) is a glycoprotein that in humans is encoded by the TNC gene. It is expressed in the extracellular matrix of various tissues during development, disease or injury, and in restricted neurogenic areas of the central nervous system. Tenascin-C is the founding member of the tenascin protein family. In the embryo it is made by migrating cells like the neural crest; it is also abundant in developing tendons, bone and cartilage.

Collagen alpha-1(XVIII) chain is a protein that in humans is encoded by the COL18A1 gene.

Laminin subunit alpha-5 is a protein that in humans is encoded by the LAMA5 gene.

Laminin subunit alpha-3 is a protein that in humans is encoded by the LAMA3 gene.

Laminin subunit alpha-1 is a protein that in humans is encoded by the LAMA1 gene.
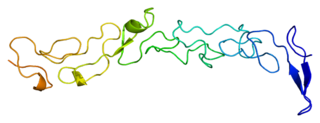
Laminin subunit gamma-1 is a protein that in humans is encoded by the LAMC1 gene.

Laminin subunit beta-1 is a protein that in humans is encoded by the LAMB1 gene.

Laminin subunit alpha-2 is a protein that in humans is encoded by the LAMA2 gene.

Collagen alpha-6(IV) chain is a protein that in humans is encoded by the COL4A6 gene.

EGF-containing fibulin-like extracellular matrix protein 1 is a protein that in humans is encoded by the EFEMP1 gene.
EGF-containing fibulin-like extracellular matrix protein 2 is a protein that in humans is encoded by the EFEMP2 gene.

Collagen alpha-1(XIII) chain is a protein that in humans is encoded by the COL13A1 gene.

Prolargin is a protein that in humans is encoded by the PRELP gene.

Nidogen-2, also known as osteonidogen, is a basal lamina protein of the nidogen family. It was the second nidogen to be described after nidogen-1 (entactin). Both play key roles during late embryonic development. In humans it is encoded by the NID2 gene.























