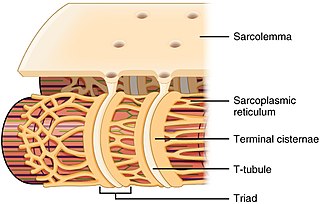
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.
SERCA, or sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, or SR Ca2+-ATPase, is a calcium ATPase-type P-ATPase. Its major function is to transport calcium from the cytosol into the sarcoplasmic reticulum.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.

Phospholamban, also known as PLN or PLB, is a micropeptide protein that in humans is encoded by the PLN gene. Phospholamban is a 52-amino acid integral membrane protein that regulates the calcium (Ca2+) pump in cardiac muscle cells.
Calsequestrin is a calcium-binding protein that acts as a calcium buffer within the sarcoplasmic reticulum. The protein helps hold calcium in the cisterna of the sarcoplasmic reticulum after a muscle contraction, even though the concentration of calcium in the sarcoplasmic reticulum is much higher than in the cytosol. It also helps the sarcoplasmic reticulum store an extraordinarily high amount of calcium ions. Each molecule of calsequestrin can bind 18 to 50 Ca2+ ions. Sequence analysis has suggested that calcium is not bound in distinct pockets via EF-hand motifs, but rather via presentation of a charged protein surface. Two forms of calsequestrin have been identified. The cardiac form Calsequestrin-2 (CASQ2) is present in cardiac and slow skeletal muscle and the fast skeletal form Calsequestrin-1(CASQ1) is found in fast skeletal muscle. The release of calsequestrin-bound calcium (through a calcium release channel) triggers muscle contraction. The active protein is not highly structured, more than 50% of it adopting a random coil conformation. When calcium binds there is a structural change whereby the alpha-helical content of the protein increases from 3 to 11%. Both forms of calsequestrin are phosphorylated by casein kinase 2, but the cardiac form is phosphorylated more rapidly and to a higher degree. Calsequestrin is also secreted in the gut where it deprives bacteria of calcium ions..

Calmodulin 1 is a protein in humans that is encoded by the CALM1 gene.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.

Calmodulin 2 is a protein that in humans is encoded by the CALM2 gene. A member of the calmodulin family of signaling molecules, it is an intermediary between calcium ions, which act as a second messenger, and many intracellular processes, such as the contraction of cardiac muscle.

Protein S100-A1, also known as S100 calcium-binding protein A1 is a protein which in humans is encoded by the S100A1 gene. S100A1 is highly expressed in cardiac and skeletal muscle, and localizes to Z-discs and sarcoplasmic reticulum. S100A1 has shown promise as an effective candidate for gene therapy to treat post-myocardially infarcted cardiac tissue.
Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 (SERCA1) also known as Calcium pump 1, is an enzyme that in humans is encoded by the ATP2A1 gene.

Cav1.1 also known as the calcium channel, voltage-dependent, L type, alpha 1S subunit, (CACNA1S), is a protein which in humans is encoded by the CACNA1S gene. It is also known as CACNL1A3 and the dihydropyridine receptor.
Obscurin is a protein that in humans is encoded by the OBSCN gene. Obscurin belongs to the family of giant sarcomeric signaling proteins that includes titin and nebulin. Obscurin is expressed in cardiac and skeletal muscle, and plays a role in the organization of myofibrils during sarcomere assembly. A mutation in the OBSCN gene has been associated with hypertrophic cardiomyopathy and altered obscurin protein properties have been associated with other muscle diseases.

Triadin, also known as TRDN, is a human gene associated with the release of calcium ions from the sarcoplasmic reticulum triggering muscular contraction through calcium-induced calcium release. Triadin is a multiprotein family, arising from different processing of the TRDN gene on chromosome 6. It is a transmembrane protein on the sarcoplasmic reticulum due to a well defined hydrophobic section and it forms a quaternary complex with the cardiac ryanodine receptor (RYR2), calsequestrin (CASQ2) and junctin proteins. The luminal (inner compartment of the sarcoplasmic reticulum) section of Triadin has areas of highly charged amino acid residues that act as luminal Ca2+ receptors. Triadin is also able to sense luminal Ca2+ concentrations by mediating interactions between RYR2 and CASQ2. Triadin has several different forms; Trisk 95 and Trisk 51, which are expressed in skeletal muscle, and Trisk 32 (CT1), which is mainly expressed in cardiac muscle.

Sarcoplasmic reticulum histidine-rich calcium-binding protein is a protein that in humans is encoded by the HRC gene.

Sarcolipin is a micropeptide protein that in humans is encoded by the SLN gene.

A-kinase anchor protein 6 is an enzyme that in humans is encoded by the AKAP6 gene.

Junctophilin 2, also known as JPH2, is a protein which in humans is encoded by the JPH2 gene. Alternative splicing has been observed at this locus and two variants encoding distinct isoforms are described.

Ryanodine receptor 1 (RYR-1) also known as skeletal muscle calcium release channel or skeletal muscle-type ryanodine receptor is one of a class of ryanodine receptors and a protein found primarily in skeletal muscle. In humans, it is encoded by the RYR1 gene.

Junctophilin-1 is a protein that in humans is encoded by the JPH1 gene.

Trimeric intracellular cation-selective channel A (TRIC-A) is a monovalent cation channel in the SR and nuclear membranes of skeletal muscle cells, encoded by the transmembrane protein 38A (TMEM38A) gene. It is one of two known TRIC proteins, the other being TRIC-B.





















