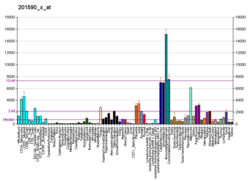
Annexin A2 also known as annexin II is a protein that in humans is encoded by the ANXA2 gene. [5]
Contents
Annexin 2 is involved in diverse cellular processes such as cell motility (especially that of the epithelial cells), linkage of membrane-associated protein complexes to the actin cytoskeleton, endocytosis, fibrinolysis, ion channel formation, and cell matrix interactions. It is a calcium-dependent phospholipid-binding protein whose function is to help organize exocytosis of intracellular proteins to the extracellular domain. Annexin II is a pleiotropic protein meaning that its function is dependent on place and time in the body.