
A tyrosine kinase is an enzyme that can transfer a phosphate group from ATP to the tyrosine residues of specific proteins inside a cell. It functions as an "on" or "off" switch in many cellular functions.

The Philadelphia chromosome or Philadelphia translocation (Ph) is a specific genetic abnormality in chromosome 22 of leukemia cancer cells. This chromosome is defective and unusually short because of reciprocal translocation, t(9;22)(q34;q11), of genetic material between chromosome 9 and chromosome 22, and contains a fusion gene called BCR-ABL1. This gene is the ABL1 gene of chromosome 9 juxtaposed onto the breakpoint cluster region BCR gene of chromosome 22, coding for a hybrid protein: a tyrosine kinase signaling protein that is "always on", causing the cell to divide uncontrollably by interrupting the stability of the genome and impairing various signaling pathways governing the cell cycle.

Chronic myelogenous leukemia (CML), also known as chronic myeloid leukemia, is a cancer of the white blood cells. It is a form of leukemia characterized by the increased and unregulated growth of myeloid cells in the bone marrow and the accumulation of these cells in the blood. CML is a clonal bone marrow stem cell disorder in which a proliferation of mature granulocytes and their precursors is found; characteristic increase in basophils is clinically relevant. It is a type of myeloproliferative neoplasm associated with a characteristic chromosomal translocation called the Philadelphia chromosome.

Imatinib, sold under the brand names Gleevec and Glivec (both marketed worldwide by Novartis) among others, is an oral targeted therapy medication used to treat cancer. Imatinib is a small molecule inhibitor targeting multiple tyrosine kinases such as CSF1R, ABL, c-KIT, FLT3, and PDGFR-β. Specifically, it is used for chronic myelogenous leukemia (CML) and acute lymphocytic leukemia (ALL) that are Philadelphia chromosome–positive (Ph+), certain types of gastrointestinal stromal tumors (GIST), hypereosinophilic syndrome (HES), chronic eosinophilic leukemia (CEL), systemic mastocytosis, and myelodysplastic syndrome.

Nilotinib, sold under the brand name Tasigna marketed worldwide by Novartis, is a medication used to treat chronic myelogenous leukemia (CML) which has the Philadelphia chromosome. It may be used both in initial cases of chronic phase CML as well as in accelerated and chronic phase CML that has not responded to imatinib. It is taken by mouth.

Dasatinib, sold under the brand name Sprycel among others, is a targeted therapy medication used to treat certain cases of chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL). Specifically it is used to treat cases that are Philadelphia chromosome-positive (Ph+). It is taken by mouth.

Acute myeloblastic leukemia with maturation (M2) is a subtype of acute myeloid leukemia (AML).
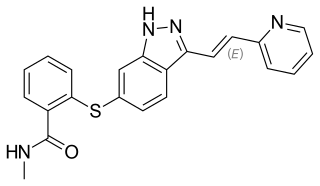
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.
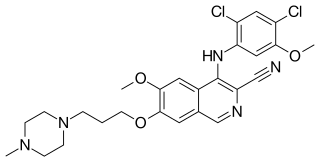
Bosutinib, sold under the brand name Bosulif, is a small molecule BCR-ABL and src tyrosine kinase inhibitor used for the treatment of chronic myelogenous leukemia.

ARIAD Pharmaceuticals, Inc. was an American oncology company, now part of Takeda Oncology, which was founded in 1991 by Harvey J. Berger, M.D. and headquartered in Cambridge, Massachusetts. ARIAD engaged in the discovery, development, and commercialization of medicines for cancer patients.

Omacetaxine mepesuccinate is a pharmaceutical drug substance that is indicated for treatment of chronic myeloid leukemia (CML).

Brian J. Druker, M.D. is a physician-scientist at Oregon Health & Science University (OHSU), in Portland, Oregon. He is the chief executive officer of OHSU's Knight Cancer Institute, JELD-WEN Chair of Leukemia Research, Associate Dean for Oncology in the OHSU School of Medicine, and professor of medicine.

Nicholas B. Lydon FRS is a British scientist and entrepreneur. In 2009, he was awarded the Lasker Clinical Award and in 2012 the Japan Prize for the development of Gleevec, also known as Imatinib, a selective BCR-ABL inhibitor for the treatment of chronic myeloid leukaemia (CML), which converted a fatal cancer into a manageable chronic condition.

A tyrosine kinase inhibitor (TKI) is a pharmaceutical drug that inhibits tyrosine kinases. Tyrosine kinases are enzymes responsible for the activation of many proteins by signal transduction cascades. The proteins are activated by adding a phosphate group to the protein (phosphorylation), a step that TKIs inhibit. TKIs are typically used as anticancer drugs. For example, they have substantially improved outcomes in chronic myelogenous leukemia. They have also been used to treat other diseases, such as idiopathic pulmonary fibrosis.
Bcr-Abl tyrosine-kinase inhibitors (TKI) are the first-line therapy for most patients with chronic myelogenous leukemia (CML). More than 90% of CML cases are caused by a chromosomal abnormality that results in the formation of a so-called Philadelphia chromosome. This abnormality was discovered by Peter Nowell in 1960 and is a consequence of fusion between the Abelson (Abl) tyrosine kinase gene at chromosome 9 and the break point cluster (Bcr) gene at chromosome 22, resulting in a chimeric oncogene (Bcr-Abl) and a constitutively active Bcr-Abl tyrosine kinase that has been implicated in the pathogenesis of CML. Compounds have been developed to selectively inhibit the tyrosine kinase.

Ponatinib, sold under the brand name Iclusig, is a medication used for the treatment of chronic myeloid leukemia and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia. It was developed by Ariad Pharmaceuticals. It is a multi-targeted tyrosine-kinase inhibitor. Some forms of chronic myeloid leukemia, those that have the T315I mutation, are resistant to current therapies such as imatinib. Ponatinib has been designed to be effective against these types of tumors.
Atypical chronic myeloid leukemia (aCML) is a type of leukemia. It is a heterogeneous disorder belonging to the group of myelodysplastic/myeloproliferative (MDS/MPN) syndromes.
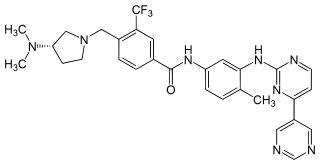
Bafetinib (NS-187) is an experimental cancer drug developed by Nippon Shinyaku and licensed to CytRx. It is an inhibitor of Lyn and Bcr-Abl. It reached phase II clinical trials in 2010.
Clonal hypereosinophilia, also termed primary hypereosinophilia or clonal eosinophilia, is a grouping of hematological disorders all of which are characterized by the development and growth of a pre-malignant or malignant population of eosinophils, a type of white blood cell that occupies the bone marrow, blood, and other tissues. This population consists of a clone of eosinophils, i.e. a group of genetically identical eosinophils derived from a sufficiently mutated ancestor cell.

Asciminib, sold under the brand name Scemblix, is a medication used to treat Philadelphia chromosome-positive chronic myeloid leukemia. Asciminib is a protein kinase inhibitor.
















