 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric |
| Target | folate receptor alpha |
| Clinical data | |
| Trade names | Elahere |
| Other names | mirvetuximab soravtansine-gynx |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a622075 |
| License data | |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| DrugBank | |
| UNII | |
| KEGG | |
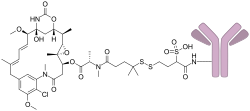
Mirvetuximab soravtansine, sold under the brand name Elahere, is a medication used as a treatment for epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. [2] [5] Mirvetuximab soravtansine is a folate receptor alpha directed antibody and microtubule inhibitor conjugate. [5] [6]
Contents
- Medical uses
- Adverse effects
- History
- Society and culture
- Legal status
- Names
- References
- Further reading
- External links
The most common adverse reactions, including laboratory abnormalities, were vision impairment, fatigue, increased aspartate aminotransferase, nausea, increased alanine aminotransferase, keratopathy, abdominal pain, decreased lymphocytes, peripheral neuropathy, diarrhea, decreased albumin, constipation, increased alkaline phosphatase, dry eye, decreased magnesium, decreased leukocytes, decreased neutrophils, and decreased hemoglobin. [5]
Mirvetuximab soravtansine was approved for medical use in the United States in November 2022. [5] [7] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [8] [9]