
Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from viricides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural viricides are produced by some plants such as eucalyptus and Australian tea trees.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.

Targeted therapy or molecularly targeted therapy is one of the major modalities of medical treatment (pharmacotherapy) for cancer, others being hormonal therapy and cytotoxic chemotherapy. As a form of molecular medicine, targeted therapy blocks the growth of cancer cells by interfering with specific targeted molecules needed for carcinogenesis and tumor growth, rather than by simply interfering with all rapidly dividing cells. Because most agents for targeted therapy are biopharmaceuticals, the term biologic therapy is sometimes synonymous with targeted therapy when used in the context of cancer therapy. However, the modalities can be combined; antibody-drug conjugates combine biologic and cytotoxic mechanisms into one targeted therapy.
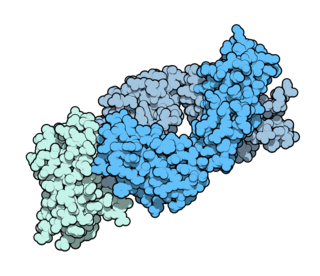
Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.

Nitazoxanide, sold under the brand name Alinia among others, is a broad-spectrum antiparasitic and broad-spectrum antiviral medication that is used in medicine for the treatment of various helminthic, protozoal, and viral infections. It is indicated for the treatment of infection by Cryptosporidium parvum and Giardia lamblia in immunocompetent individuals and has been repurposed for the treatment of influenza. Nitazoxanide has also been shown to have in vitro antiparasitic activity and clinical treatment efficacy for infections caused by other protozoa and helminths; evidence as of 2014 suggested that it possesses efficacy in treating a number of viral infections as well.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania
Mapatumumab (HGS-ETR1) is an experimental human monoclonal antibody undergoing clinical trials for the treatment of cancer. It targets TRAIL-R1, also known as DR4, which is expressed on the surface of many tumor cell types.
Ramucirumab is a fully human monoclonal antibody (IgG1) developed for the treatment of solid tumors. This drug was developed by ImClone Systems Inc. It was isolated from a native phage display library from Dyax.
Treatment of lung cancer refers to the use of medical therapies, such as surgery, radiation, chemotherapy, immunotherapy, percutaneous ablation, and palliative care, alone or in combination, in an attempt to cure or lessen the adverse impact of malignant neoplasms originating in lung tissue.

ALK inhibitors are anti-cancer drugs that act on tumours with variations of anaplastic lymphoma kinase (ALK) such as an EML4-ALK translocation. They fall under the category of tyrosine kinase inhibitors, which work by inhibiting proteins involved in the abnormal growth of tumour cells. All the current approved ALK inhibitors function by binding to the ATP pocket of the abnormal ALK protein, blocking its access to energy and deactivating it. A majority of ALK-rearranged NSCLC harbour the EML4-ALK fusion, although as of 2020, over 92 fusion partners have been discovered in ALK+ NSCLC. For each fusion partner, there can be several fusion variants depending on the position the two genes were fused at, and this may have implications on the response of the tumour and prognosis of the patient.

Selumetinib (INN), sold under the brand name Koselugo, is a medication for the treatment of children, two years of age and older, with neurofibromatosis type I (NF-1), a genetic disorder of the nervous system causing tumors to grow on nerves. It is taken by mouth.

Brigatinib, sold under the brand name Alunbrig among others, is a small-molecule targeted cancer therapy being developed by Ariad Pharmaceuticals, Inc. Brigatinib acts as both an anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitor.
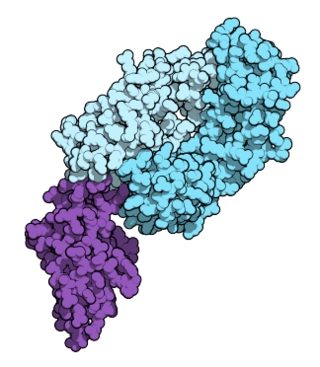
Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.
Targeted molecular therapy for neuroblastoma involves treatment aimed at molecular targets that have a unique expression in this form of cancer. Neuroblastoma, the second most common pediatric malignant tumor, often involves treatment through intensive chemotherapy. A number of molecular targets have been identified for the treatment of high-risk forms of this disease. Aiming treatment in this way provides a more selective way to treat the disease, decreasing the risk for toxicities that are associated with the typical treatment regimen. Treatment using these targets can supplement or replace some of the intensive chemotherapy that is used for neuroblastoma. These molecular targets of this disease include GD2, ALK, and CD133. GD2 is a target of immunotherapy, and is the most fully developed of these treatment methods, but is also associated with toxicities. ALK has more recently been discovered, and drugs in development for this target are proving to be successful in neuroblastoma treatment. The role of CD133 in neuroblastoma has also been more recently discovered and is an effective target for treatment of this disease.
A MEK inhibitor is a chemical or drug that inhibits the mitogen-activated protein kinase kinase enzymes MEK1 and/or MEK2. They can be used to affect the MAPK/ERK pathway which is often overactive in some cancers.

Atezolizumab, sold under the brand name Tecentriq, is a monoclonal antibody medication used to treat urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer (TNBC), small cell lung cancer (SCLC), hepatocellular carcinoma, and alveolar soft part sarcoma. It is a fully humanized, engineered monoclonal antibody of IgG1 isotype against the protein programmed cell death-ligand 1 (PD-L1).

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.
RNA therapeutics are a new class of medications based on ribonucleic acid (RNA). Research has been working on clinical use since the 1990s, with significant success in cancer therapy in the early 2010s. In 2020 and 2021, mRNA vaccines have been developed globally for use in combating the coronavirus disease. The Pfizer–BioNTech COVID-19 vaccine was the first mRNA vaccine approved by a medicines regulator, followed by the Moderna COVID-19 vaccine, and others.
Roy S. Herbst is an American oncologist who is the Ensign Professor of Medicine, Professor of Pharmacology, Chief of Medical Oncology, and Associate Director for Translational Research at Yale Cancer Center and Yale School of Medicine in New Haven, Connecticut.
Amivantamab, sold under the brand name Rybrevant, is a bispecific monoclonal antibody used to treat non-small cell lung cancer. Amivantamab is a bispecific epidermal growth factor (EGF) receptor-directed and mesenchymal–epithelial transition (MET) receptor-directed antibody. It is the first treatment for adults with non-small cell lung cancer whose tumors have specific types of genetic mutations: epidermal growth factor receptor (EGFR) exon 20 insertion mutations.










