Related Research Articles

Astatine is a chemical element; it has symbol At and atomic number 85. It is the rarest naturally occurring element in the Earth's crust, occurring only as the decay product of various heavier elements. All of astatine's isotopes are short-lived; the most stable is astatine-210, with a half-life of 8.1 hours. Consequently, a solid sample of the element has never been seen, because any macroscopic specimen would be immediately vaporized by the heat of its radioactivity.

Hyperthyroidism is the condition that occurs due to excessive production of thyroid hormones by the thyroid gland. Thyrotoxicosis is the condition that occurs due to excessive thyroid hormone of any cause and therefore includes hyperthyroidism. Some, however, use the terms interchangeably. Signs and symptoms vary between people and may include irritability, muscle weakness, sleeping problems, a fast heartbeat, heat intolerance, diarrhea, enlargement of the thyroid, hand tremor, and weight loss. Symptoms are typically less severe in the elderly and during pregnancy. An uncommon but life-threatening complication is thyroid storm in which an event such as an infection results in worsening symptoms such as confusion and a high temperature; this often results in death. The opposite is hypothyroidism, when the thyroid gland does not make enough thyroid hormone.

Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at 114 °C (237 °F), and boils to a violet gas at 184 °C (363 °F). The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek Ιώδης 'violet-coloured'.

Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name biotin, borrowed from the German Biotin, derives from the Ancient Greek word βίοτος (bíotos; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white crystalline solid that looks like needles.

Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used. In the third world it is also used for treating skin sporotrichosis and phycomycosis. It is a supplement used by people with low dietary intake of iodine. It is administered orally.
131 is the natural number following 130 and preceding 132.

Lugol's iodine, also known as aqueous iodine and strong iodine solution, is a solution of potassium iodide with iodine in water. It is a medication and disinfectant used for a number of purposes. Taken by mouth it is used to treat thyrotoxicosis until surgery can be carried out, protect the thyroid gland from radioactive iodine, and to treat iodine deficiency. When applied to the cervix it is used to help in screening for cervical cancer. As a disinfectant it may be applied to small wounds such as a needle stick injury. A small amount may also be used for emergency disinfection of drinking water.

Streptavidin is a 52 kDa protein (tetramer) purified from the bacterium Streptomyces avidinii. Streptavidin homo-tetramers have an extraordinarily high affinity for biotin. With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants, detergents, proteolytic enzymes, and extremes of temperature and pH.

Iodine-131 is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission. See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium.

Periodic acid is the highest oxoacid of iodine, in which the iodine exists in oxidation state +7. It can exist in two forms: orthoperiodic acid, with the chemical formula H5IO6, and metaperiodic acid, which has the formula HIO4.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.
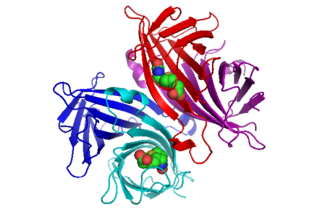
Avidin is a tetrameric biotin-binding protein produced in the oviducts of birds, reptiles and amphibians and deposited in the whites of their eggs. Dimeric members of the avidin family are also found in some bacteria. In chicken egg white, avidin makes up approximately 0.05% of total protein (approximately 1800 μg per egg). The tetrameric protein contains four identical subunits (homotetramer), each of which can bind to biotin (Vitamin B7, vitamin H) with a high degree of affinity and specificity. The dissociation constant of the avidin-biotin complex is measured to be KD ≈ 10−15 M, making it one of the strongest known non-covalent bonds.
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.
Iodine-123 (123I) is a radioactive isotope of iodine used in nuclear medicine imaging, including single-photon emission computed tomography (SPECT) or SPECT/CT exams. The isotope's half-life is 13.2230 hours; the decay by electron capture to tellurium-123 emits gamma radiation with a predominant energy of 159 keV. In medical applications, the radiation is detected by a gamma camera. The isotope is typically applied as iodide-123, the anionic form.
Tositumomab is a murine monoclonal antibody which targets the CD20 antigen produced in mammalian cell. It was combined with iodine-131 to produce a radiopharmaceutical for unsealed source radiotherapy, Iodine-131 Tositumomab, for the treatment of non-Hodgkins lymphoma. It is classified as a IgG2a lambda antibody.
In enzymology, a biotin—[acetyl-CoA-carboxylase] ligase is an enzyme that catalyzes the chemical reaction
3F8 is a murine IgG3 monoclonal antibody which binds to GD2.
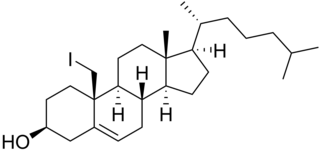
Iodocholesterol, or 19-iodocholesterol, also as iodocholesterol (131I) (INN) and NP-59, is a derivative of cholesterol with an iodine atom in the C19 position and a radiopharmaceutical. When the iodine atom is a radioactive isotope, it is used as an adrenal cortex radiotracer in the diagnosis of patients suspected of having Cushing's syndrome, hyperaldosteronism, pheochromocytoma, and adrenal remnants following total adrenalectomy.

Paul György (April 7, 1893 – March 1, 1976) was a Hungarian-born American biochemist, nutritionist, and pediatrician best known for his discovery of three B vitamins: riboflavin, B6, and biotin. Gyorgy was also well known for his research into the protective factors of human breast milk, particularly for his discoveries of Lactobacillus bifidus growth factor activity in human milk and its anti-staphylococcal properties. He was a recipient of the National Medal of Science in 1975 from President Gerald Ford.
A medical isotope is an isotope used in medicine.