
Technetium (99mTc) sestamibi (INN) is a pharmaceutical agent used in nuclear medicine imaging. The drug is a coordination complex consisting of the radioisotope technetium-99m bound to six (sesta=6) methoxyisobutylisonitrile (MIBI) ligands. The anion is not defined. The generic drug became available late September 2008. A scan of a patient using MIBI is commonly known as a "MIBI scan".
This is a list of terms related to oncology. The original source for this list was the US National Cancer Institute's public domain Dictionary of Cancer Terms.

Hybridoma technology is a method for producing large numbers of identical antibodies, also called monoclonal antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal myeloma cancer cells, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma.

Carcinoembryonic antigen (CEA) describes a set of highly-related glycoproteins involved in cell adhesion. CEA is normally produced in gastrointestinal tissue during fetal development, but the production stops before birth. Consequently, CEA is usually present at very low levels in the blood of healthy adults. However, the serum levels are raised in some types of cancer, which means that it can be used as a tumor marker in clinical tests. Serum levels can also be elevated in heavy smokers.

Radioimmunotherapy (RIT) uses an antibody labeled with a radionuclide to deliver cytotoxic radiation to a target cell. It is a form of unsealed source radiotherapy. In cancer therapy, an antibody with specificity for a tumor-associated antigen is used to deliver a lethal dose of radiation to the tumor cells. The ability for the antibody to specifically bind to a tumor-associated antigen increases the dose delivered to the tumor cells while decreasing the dose to normal tissues. By its nature, RIT requires a tumor cell to express an antigen that is unique to the neoplasm or is not accessible in normal cells.
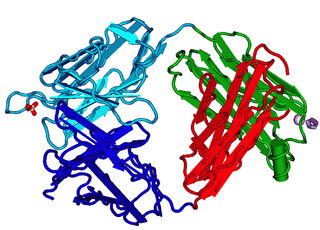
The fragment antigen-binding region is a region on an antibody that binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. The variable domain contains the paratope, comprising a set of complementarity-determining regions, at the amino terminal end of the monomer. Each arm of the Y thus binds an epitope on the antigen.
Technetium (99mTc) fanolesomab is a mouse monoclonal antibody formerly used to aid in the diagnosis of appendicitis. It is labeled with a radioisotope, technetium-99m (99mTc).
Technetium (99mTc) sulesomab is a radio-pharmaceutical composed of anti-human mouse monoclonal antibody that targets the granulocyte associated NCA-90 cell antigen and a conjugated technetium-99m radionuclide. After intravenous administration, Leukoscan enables sensitive and specific whole body measurement of granulocyte infiltration and activation by gamma camera imaging of 99mTc-antibody bound cells. Total clearance of LeukoScan from blood samples after administration and imaging has been reported at 48 hour time points indicating limited retention of the agent in circulation

Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99, symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medical radioisotope in the world.
Bectumomab is a mouse monoclonal antibody labelled with the radioactive isotope technetium-99m. It is used to detect non-Hodgkin's lymphoma.
Labetuzumab is a humanized IgG1 monoclonal antibody for the treatment of colorectal cancer. It selectively binds to carcinoembryonic cell adhesion molecule 5.

Technetium (99mTc) nofetumomab merpentan is a mouse monoclonal antibody derivative used in the diagnosis of lung cancer, gastrointestinal, breast, ovary, pancreas, kidney, cervix, and bladder carcinoma. The antibody part, nofetumomab, is attached to the chelator merpentan, which links it to the radioisotope technetium-99m (99mTc).
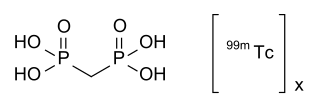
Technetium (99mTc) medronic acid is a pharmaceutical product used in nuclear medicine to localize bone metastases as well as other diseases that can alter the natural turn-over in the bone by bone scintigraphy.
Tumor-associated glycoprotein 72 (TAG-72) is a glycoprotein found on the surface of many cancer cells, including ovary, breast, colon, lung, and pancreatic cancers. It is a mucin-like molecule with a molar mass of over 1000 kDa.

Carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) also known as CD66e, is a member of the carcinoembryonic antigen (CEA) gene family.
Immunoscintigraphy is a nuclear medicine procedure used to find cancer cells in the body by injecting a radioactively labeled antibody, which binds predominantly to cancer cells and then scanning for concentrations of radioactive emissions.
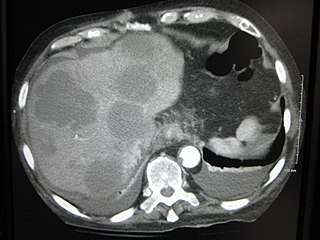
A liver metastasis is a malignant tumor in the liver that has spread from another organ affected by cancer. The liver is a common site for metastatic disease because of its rich, dual blood supply. Metastatic tumors in the liver are 20 times more common than primary tumors. In 50% of all cases the primary tumor is of the gastrointestinal tract; other common sites include the breast, ovaries, bronchus and kidney. Patients with colorectal cancer may also develop liver metastases.
Technetium (99mTc) votumumab is a human monoclonal antibody labelled with the radionuclide technetium-99m. It was developed for the detection of colorectal tumors, but has never been marketed.
Milatuzumab is an anti-CD74 humanized monoclonal antibody for the treatment of multiple myeloma non-Hodgkin's lymphoma and chronic lymphocytic leukemia.
Immunomedics was a biotechnology company focused on the development of antibody-drug conjugates for the treatment of cancer. In 2020, the company was acquired by Gilead Sciences.








