
Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer, improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.

Sorafenib, sold under the brand name Nexavar, is a kinase inhibitor drug approved for the treatment of primary kidney cancer, advanced primary liver cancer, FLT3-ITD positive AML and radioactive iodine resistant advanced thyroid carcinoma.

Sunitinib, sold under the brand name Sutent, is an anti-cancer medication. It is a small-molecule, multi-targeted receptor tyrosine kinase (RTK) inhibitor that was approved by the FDA for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST) in January 2006. Sunitinib was the first cancer drug simultaneously approved for two different indications.
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.
Bavituximab (PGN401) is a human-mouse chimeric monoclonal antibody against phosphatidylserine, which is a component of cell membranes that is exposed when a cell is transformed into solid tumor cancer cell or dies, and when cells are infected with hepatitis C. The process of cell death is highly controlled and so there usually no immune response to phosphatidylserine but when bavituximab binds to it, the conjugate appears to stimulate an immune response in humans.
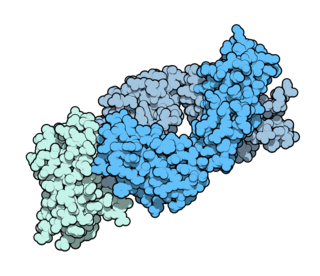
Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4, a protein receptor that downregulates the immune system.
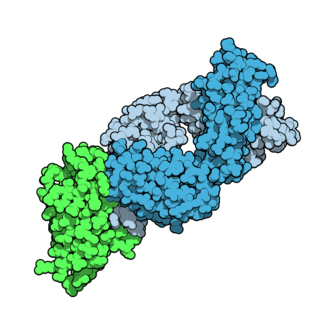
Tremelimumab, sold under the brand name Imjudo, is a fully human monoclonal antibody used for the treatment of hepatocellular carcinoma. Tremelimumab is designed to attach to and block CTLA-4, a protein that controls the activity of T cells, which are part of the immune system.
Matuzumab is a humanized monoclonal antibody for the treatment of cancer. It binds to the epidermal growth factor receptor (EGFR) with high affinity. The mouse monoclonal antibody (mAb425) from which matuzumab was developed at the Wistar Institute in Philadelphia, Pennsylvania
Nimotuzumab is a humanized monoclonal antibody that as of 2014 had orphan status in the US and EU for glioma, and marketing approval in India, China, and other countries for squamous cell carcinomas of the head and neck, and was undergoing several clinical trials.
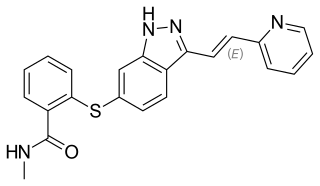
Axitinib, sold under the brand name Inlyta, is a small molecule tyrosine kinase inhibitor developed by Pfizer. It has been shown to significantly inhibit growth of breast cancer in animal (xenograft) models and has shown partial responses in clinical trials with renal cell carcinoma (RCC) and several other tumour types.
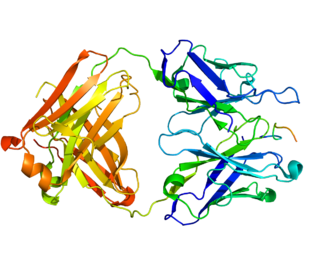
Carbonic anhydrase IX is an enzyme that in humans is encoded by the CA9 gene. It is one of the 14 carbonic anhydrase isoforms found in humans and is a transmembrane dimeric metalloenzyme with an extracellular active site that facilitates acid secretion in the gastrointestinal tract. CA IX is overexpressed in many types of cancer including clear cell renal cell carcinoma (RCC) as well as carcinomas of the cervix, breast and lung where it promotes tumor growth by enhancing tumor acidosis.

Lloyd John Old was one of the founders and standard-bearers of the field of cancer immunology. When Old began his career in 1958, tumor immunology was in its infancy. Today, cancer immunotherapies are emerging as a significant advance in cancer therapy.
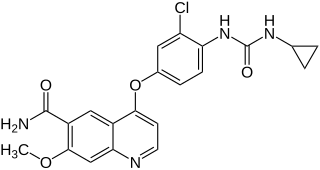
Lenvatinib, sold under the brand name Lenvima among others, is an anti-cancer medication for the treatment of certain kinds of thyroid cancer and for other cancers as well. It was developed by Eisai Co. and acts as a multiple kinase inhibitor against the VEGFR1, VEGFR2 and VEGFR3 kinases.
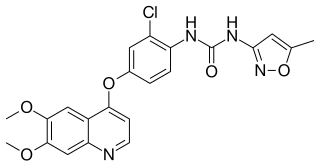
Tivozanib, sold under the brand name Fotivda, is a medication used for the treatment of advanced renal cell carcinoma. It is an oral VEGF receptor tyrosine kinase inhibitor.

Zoptarelin doxorubicin consists of doxorubicin linked to a small peptide agonist to the luteinizing hormone-releasing hormone (LHRH) receptor. It has been developed as a potential treatment for a number of human cancers. The LHRH receptor is aberrantly present on the cell surface of approximately 80% of endometrial and ovarian cancers, 86% of prostate cancers and about 50% of breast cancers. Whereas in normal tissues, expression of this receptor is mainly confined to the pituitary gland, reproductive organs and hematopoietic stem cells. To a lesser extent the LHRH receptor is also found on the surface of bladder, colorectal, and pancreatic cancers, sarcomas, lymphomas, melanomas, and renal cell carcinomas.
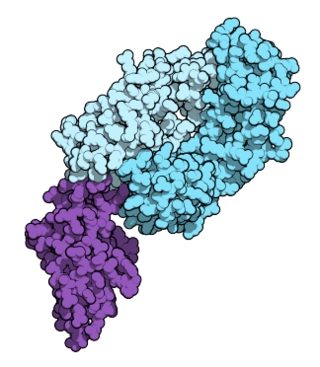
Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is administered by slow intravenous injection.
Seagen Inc. is an American biotechnology company focused on developing and commercializing innovative, empowered monoclonal antibody-based therapies for the treatment of cancer. The company, headquartered in Bothell, Washington, is the industry leader in antibody-drug conjugates or ADCs, a technology designed to harness the targeting ability of monoclonal antibodies to deliver cell-killing agents directly to cancer cells. Antibody-drug conjugates are intended to spare non-targeted cells and thus reduce many of the toxic effects of traditional chemotherapy, while potentially enhancing antitumor activity.
Avelumab, sold under the brand name Bavencio, is a fully human monoclonal antibody medication for the treatment of Merkel cell carcinoma, urothelial carcinoma, and renal cell carcinoma.

PD-1 inhibitors and PD-L1 inhibitors are a group of checkpoint inhibitor anticancer drugs that block the activity of PD-1 and PDL1 immune checkpoint proteins present on the surface of cells. Immune checkpoint inhibitors are emerging as a front-line treatment for several types of cancer.













