Related Research Articles
An international nonproprietary name (INN) is an official generic and non-proprietary name given to a pharmaceutical drug or an active ingredient. INNs are intended to make communication more precise by providing a unique standard name for each active ingredient, to avoid prescribing errors. The INN system has been coordinated by the World Health Organization (WHO) since 1953.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.
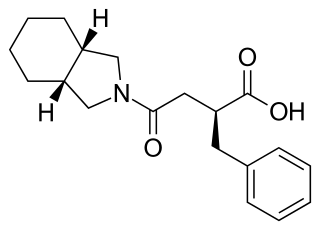
Mitiglinide is a drug for the treatment of type 2 diabetes.

Acefylline (INN), also known as acetyloxytheophylline, is a stimulant drug of the xanthine chemical class. It acts as an adenosine receptor antagonist. It is combined with diphenhydramine in the pharmaceutical preparation etanautine to help offset diphenhydramine induced drowsiness.

Drug nomenclature is the systematic naming of drugs, especially pharmaceutical drugs. In the majority of circumstances, drugs have 3 types of names: chemical names, the most important of which is the IUPAC name; generic or nonproprietary names, the most important of which are the International Nonproprietary Names (INNs); and trade names, which are brand names. Generic names for drugs are nowadays constructed out of affixes and stems that classify the drugs into different categories and also separate drugs within categories. A marketed drug might also have a company code or compound code.
Imgatuzumab (INN) is a humanized monoclonal antibody designed for the treatment of cancer. It acts as an immunomodulator.
Perakizumab (INN) is a humanized monoclonal antibody designed for the treatment of arthritis. It binds to IL17A and acts as an immunomodulator.
Lulizumab pegol is a monoclonal antibody designed for the treatment of autoimmune diseases.
Fletikumab (NNC0109-0012) (INN) is a monoclonal antibody designed for the treatment of rheumatoid arthritis.
Ulocuplumab is a monoclonal antibody designed for the treatment of hematologic malignancies.
Idarucizumab, sold under the brand name Praxbind, is a monoclonal antibody used as a reversal agent for dabigatran.
Seribantumab is a monoclonal antibody designed for the treatment of cancer.
Plozalizumab is a humanized monoclonal antibody designed for the treatment of diabetic nephropathy and arteriovenous graft patency.
Vobarilizumab is a humanized monoclonal antibody designed for the treatment of inflammatory autoimmune diseases.
Brazikumab is a human monoclonal antibody designed for the treatment of Crohn's disease.
Bleselumab is a human monoclonal antibody designed for the prevention of organ transplant rejection.
Carotuximab (INN) (TRC-105) is a chimeric monoclonal antibody designed for the treatment of cancer.
Pamrevlumab is a humanized monoclonal antibody designed for the treatment of idiopathic pulmonary fibrosis and pancreatic cancer.
Trevogrumab is a human monoclonal antibody designed for the treatment of muscle atrophy due to orthopedic disuse and sarcopenia.
References
| This monoclonal antibody–related article is a stub. You can help Wikipedia by expanding it. |