
Multiple myeloma (MM), also known as plasma cell myeloma and simply myeloma, is a cancer of plasma cells, a type of white blood cell that normally produces antibodies. Often, no symptoms are noticed initially. As it progresses, bone pain, anemia, renal insuficiency, and infections may occur. Complications may include hypercalcemia and amyloidosis.

Lenalidomide, sold under the brand name Revlimid among others, is a medication used to treat multiple myeloma, smoldering myeloma, and myelodysplastic syndromes (MDS). For multiple myeloma, it is a first line treatment, and is given with dexamethasone. It is taken by mouth.

Bortezomib, sold under the brand name Velcade among others, is an anti-cancer medication used to treat multiple myeloma and mantle cell lymphoma. This includes multiple myeloma in those who have and have not previously received treatment. It is generally used together with other medications. It is given by injection.

Celgene Corporation is a pharmaceutical company that makes cancer and immunology drugs. Its major product is Revlimid (lenalidomide), which is used in the treatment of multiple myeloma, and also in certain anemias. The company is incorporated in Delaware, headquartered in Summit, New Jersey, and a subsidiary of Bristol Myers Squibb (BMS).

Pomalidomide, sold under the brand names Pomalyst and Imnovid, is an anti-cancer medication used for the treatment of multiple myeloma and AIDS-related Kaposi sarcoma.

Carfilzomib, sold under the brand name Kyprolis, is an anti-cancer medication acting as a selective proteasome inhibitor. Chemically, it is a tetrapeptide epoxyketone and an analog of epoxomicin. It was developed by Onyx Pharmaceuticals.

Indatuximab ravtansine (BT062) is an immunomodulator and antineoplastic antibody-drug conjugate.

Daratumumab, sold under the brand name Darzalex among others, is an anti-cancer monoclonal antibody medication. It binds to CD38, which is overexpressed in multiple myeloma cells. Daratumumab was originally developed by Genmab, but it is now being jointly developed by Genmab along with the Johnson & Johnson subsidiary Janssen Biotech, which acquired worldwide commercialization rights to the drug from Genmab.

Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

Cereblon E3 ligase modulators, also known as immunomodulatory imide drugs (IMiDs), are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues. These drugs may also be referred to as 'Cereblon modulators'. Cereblon (CRBN) is the protein targeted by this class of drugs.
MDX-1097 is a monoclonal antibody therapy that in 2023 has been assessed in a Phase IIb clinical trial in conjunction with lenalidomide and dexamethasone as a treatment for multiple myeloma, a type of white blood cell cancer. MDX-1097 was originally developed by scientists at Immune System Therapeutics Ltd. In 2015, Haemalogix Ltd acquired the rights to MDX-1097 and are taking it through clinical testing.

Isatuximab, sold under the brand name Sarclisa, is a monoclonal antibody (mAb) medication for the treatment of multiple myeloma.

Ixazomib is a drug for the treatment of multiple myeloma, a type of white blood cell cancer, in combination with other drugs. It is taken by mouth in the form of capsules.

Melphalan flufenamide, sold under the brand names Pepaxto and Pepaxti, is an anticancer medication used to treat multiple myeloma.

Selinexor sold under the brand name Xpovio among others, is a selective inhibitor of nuclear export used as an anti-cancer medication. It works by blocking the action of exportin 1 and thus blocking the transport of several proteins involved in cancer-cell growth from the cell nucleus to the cytoplasm, which ultimately arrests the cell cycle and leads to apoptosis. It is the first drug with this mechanism of action.
Daratumumab/hyaluronidase, sold under the brand name Darzalex Faspro, is a fixed-dose combination medication for the treatment of adults with newly diagnosed or relapsed/refractory multiple myeloma. It is a combination of daratumumab and hyaluronidase. It is administered via subcutaneous injection.
Belantamab mafodotin, sold under the brand name Blenrep, is a medication for the treatment of relapsed and refractory multiple myeloma.
Idecabtagene vicleucel, sold under the brand name Abecma, is a cell-based gene therapy to treat multiple myeloma.
Ciltacabtagene autoleucel, sold under the brand name Carvykti, is an anti-cancer medication used to treat multiple myeloma. Ciltacabtagene autoleucel is a BCMA -directed genetically modified autologous chimeric antigen receptor (CAR) T-cell therapy. Each dose is customized using the recipient's own T-cells, which are collected and genetically modified, and infused back into the recipient.
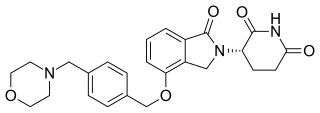
Iberdomide is an experimental thalidomide analog that works as an cereblon E3 ligase modulator; it has a higher binding affinity than lenalidomide or pomalidomide. It is developed by Bristol Myers Squibb for various cancers and was also tested in people with lupus.














