
Bladder cancer is any of several types of cancer arising from the tissues of the urinary bladder. Symptoms include blood in the urine, pain with urination, and low back pain. It is caused when epithelial cells that line the bladder become malignant.
This is a list of terms related to oncology. The original source for this list was the US National Cancer Institute's public domain Dictionary of Cancer Terms.
A gallium scan is a type of nuclear medicine test that uses either a gallium-67 (67Ga) or gallium-68 (68Ga) radiopharmaceutical to obtain images of a specific type of tissue, or disease state of tissue. Gallium salts like gallium citrate and gallium nitrate may be used. The form of salt is not important, since it is the freely dissolved gallium ion Ga3+ which is active. Both 67Ga and 68Ga salts have similar uptake mechanisms. Gallium can also be used in other forms, for example 68Ga-PSMA is used for cancer imaging. The gamma emission of gallium-67 is imaged by a gamma camera, while the positron emission of gallium-68 is imaged by positron emission tomography (PET).

Glutamate carboxypeptidase II (GCPII), also known as N-acetyl-L-aspartyl-L-glutamate peptidase I, NAAG peptidase, or prostate-specific membrane antigen (PSMA) is an enzyme that in humans is encoded by the FOLH1 gene. Human GCPII contains 750 amino acids and weighs approximately 84 kDa.
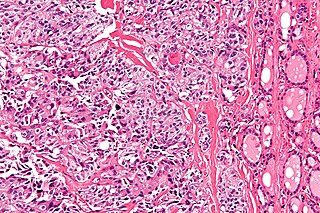
Medullary thyroid cancer is a form of thyroid carcinoma which originates from the parafollicular cells, which produce the hormone calcitonin. Medullary tumors are the third most common of all thyroid cancers and together make up about 3% of all thyroid cancer cases. MTC was first characterized in 1959.
Sipuleucel-T, sold under the brand name Provenge, developed by Dendreon Pharmaceuticals, LLC, is a cell-based cancer immunotherapy for prostate cancer (CaP). It is an autologous cellular immunotherapy.
Cervical cancer staging is the assessment of cervical cancer to determine the extent of the disease. This is important for determining disease prognosis and treatment. Cancer staging generally runs from stage 0, which is pre-cancerous or non-invasive, to stage IV, in which the cancer has spread throughout a significant part of the body.

Nivolumab, sold under the brand name Opdivo, is a medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction (GEJ) cancer. It is used by slow injection into a vein.
Advanced Accelerator Applications is a France-based pharmaceutical group, specialized in the field of nuclear medicine. The group operates in all three segments of nuclear medicine to diagnose and treat serious conditions in the fields of oncology, neurology, cardiology, infectious and inflammatory diseases.

Fluciclovine (18F), also known as anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid, or as Axumin, is a diagnostic agent indicated for positron emission tomography (PET) imaging in men with suspected prostate cancer recurrence based on elevated prostate specific antigen (PSA) levels.

Capmatinib, sold under the brand name Tabrecta, is an anticancer medication used for the treatment of metastatic non-small cell lung cancer whose tumors have a mutation that leads to the exon 14 skipping of the MET gene, which codes for the membrane receptor HGFR.
A PSMA scan is a nuclear medicine imaging technique used in the diagnosis and staging of prostate cancer. It is carried out by injection of a radiopharmaceutical with a positron or gamma emitting radionuclide and a prostate-specific membrane antigen (PSMA) targeting ligand. After injection, imaging of positron emitters such as gallium-68 (68Ga), copper-64 (64Cu), and fluorine-18 (18F) is carried out with a positron emission tomography (PET) scanner. For gamma emitters such as technetium-99m (99mTc) and indium-111 (111In) single-photon emission computed tomography (SPECT) imaging is performed with a gamma camera.

Carbon-11 choline is the basis of medical imaging technologies. Because of its involvement in biologic processes, choline is related to diseases, leading to the development of medical imaging techniques to monitor its concentration. When radiolabeled with 11CH3, choline is a useful a tracer in PET imaging. Carbon-11 is radioactive with a half-life of 20.38 minutes. By monitoring the gamma radiation resulting from the decay of carbon-11, the uptake, distribution, and retention of carbon-11 choline can be monitored.
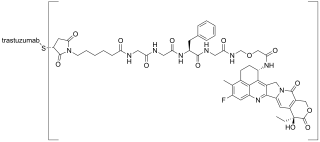
Trastuzumab deruxtecan, sold under the brand name Enhertu, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the topoisomerase I inhibitor deruxtecan. It is licensed for the treatment of breast cancer or gastric or gastroesophageal adenocarcinoma. Trastuzumab binds to and blocks signaling through epidermal growth factor receptor 2 (HER2/neu) on cancers that rely on it for growth. Additionally, once bound to HER2 receptors, the antibody is internalized by the cell, carrying the bound deruxtecan along with it, where it interferes with the cell's ability to make DNA structural changes and replicate its DNA during cell division, leading to DNA damage when the cell attempts to replicate itself, destroying the cell.

Ripretinib, sold under the brand name Qinlock, is a medication for the treatment of adults with advanced gastrointestinal stromal tumor (GIST), a type of tumor that originates in the gastrointestinal tract. It is taken by mouth. Ripretinib inhibits the activity of the kinases KIT and PDGFRA, which helps keep cancer cells from growing.

Flortaucipir (18F), sold under the brand name Tauvid, is a radioactive diagnostic agent indicated for use with positron emission tomography (PET) imaging to image the brain.
Brexucabtagene autoleucel, sold under the brand name Tecartus, is a cell-based gene therapy medication for the treatment of mantle cell lymphoma (MCL) and acute lymphoblastic leukemia (ALL).

Gallium (68Ga) gozetotide or Gallium (68Ga) PSMA-11 sold under the brand name Illuccix among others, is a radiopharmaceutical made of 68Ga conjugated to prostate-specific membrane antigen (PSMA) targeting ligand, Glu-Urea-Lys(Ahx)-HBED-CC, used for imaging prostate cancer by positron emission tomography (PET). The PSMA targeting ligand specifically directs the radiolabeled imaging agent towards the prostate cancerous lesions in men.

Lutetium (177Lu) vipivotide tetraxetan, sold under the brand name Pluvicto, is a radiopharmaceutical medication used for the treatment of prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC). Lutetium (177Lu) vipivotide tetraxetan is a targeted radioligand therapy.
Flotufolastat F-18, sold under the brand name Posluma, is a radioactive diagnostic agent for use with positron emission tomography (PET) imaging for prostate cancer. The active ingredient is flotufolastat F-18 gallium.












