Magnetic resonance imaging (MRI) is a medical imaging technique used in radiology to form pictures of the anatomy and the physiological processes inside the body. MRI scanners use strong magnetic fields, magnetic field gradients, and radio waves to generate images of the organs in the body. MRI does not involve X-rays or the use of ionizing radiation, which distinguishes it from computed tomography (CT) and positron emission tomography (PET) scans. MRI is a medical application of nuclear magnetic resonance (NMR) which can also be used for imaging in other NMR applications, such as NMR spectroscopy.
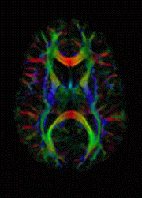
Diffusion-weighted magnetic resonance imaging is the use of specific MRI sequences as well as software that generates images from the resulting data that uses the diffusion of water molecules to generate contrast in MR images. It allows the mapping of the diffusion process of molecules, mainly water, in biological tissues, in vivo and non-invasively. Molecular diffusion in tissues is not random, but reflects interactions with many obstacles, such as macromolecules, fibers, and membranes. Water molecule diffusion patterns can therefore reveal microscopic details about tissue architecture, either normal or in a diseased state. A special kind of DWI, diffusion tensor imaging (DTI), has been used extensively to map white matter tractography in the brain.
Magnetic resonance elastography (MRE) is a form of elastography that specifically leverages MRI to quantify and subsequently map the mechanical properties of soft tissue. First developed and described at Mayo Clinic by Muthupillai et al. in 1995, MRE has emerged as a powerful, non-invasive diagnostic tool, namely as an alternative to biopsy and serum tests for staging liver fibrosis.
During nuclear magnetic resonance observations, spin–lattice relaxation is the mechanism by which the longitudinal component of the total nuclear magnetic moment vector (parallel to the constant magnetic field) exponentially relaxes from a higher energy, non-equilibrium state to thermodynamic equilibrium with its surroundings (the "lattice"). It is characterized by the spin–lattice relaxation time, a time constant known as T1.
Kenneth Kin Man Kwong is a Hong Kong-born American nuclear physicist. He is a pioneer in human brain imaging. He received his bachelor's degree in Political Science in 1972 from the University of California, Berkeley. He went on to receive his Ph.D. in physics from the University of California, Riverside studying photon-photon collision interactions.
MRI contrast agents are contrast agents used to improve the visibility of internal body structures in magnetic resonance imaging (MRI). The most commonly used compounds for contrast enhancement are gadolinium-based contrast agents (GBCAs). Such MRI contrast agents shorten the relaxation times of nuclei within body tissues following oral or intravenous administration.
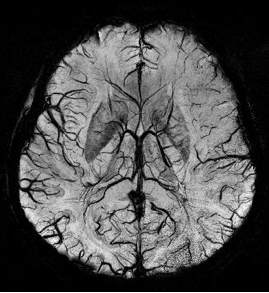
Susceptibility weighted imaging (SWI), originally called BOLD venographic imaging, is an MRI sequence that is exquisitely sensitive to venous blood, hemorrhage and iron storage. SWI uses a fully flow compensated, long echo, gradient recalled echo (GRE) pulse sequence to acquire images. This method exploits the susceptibility differences between tissues and uses the phase image to detect these differences. The magnitude and phase data are combined to produce an enhanced contrast magnitude image. The imaging of venous blood with SWI is a blood-oxygen-level dependent (BOLD) technique which is why it was referred to as BOLD venography. Due to its sensitivity to venous blood SWI is commonly used in traumatic brain injuries (TBI) and for high resolution brain venographies but has many other clinical applications. SWI is offered as a clinical package by Philips and Siemens but can be run on any manufacturer's machine at field strengths of 1.0 T, 1.5 T, 3.0 T and higher.

Magnetic resonance neurography (MRN) is the direct imaging of nerves in the body by optimizing selectivity for unique MRI water properties of nerves. It is a modification of magnetic resonance imaging. This technique yields a detailed image of a nerve from the resonance signal that arises from in the nerve itself rather than from surrounding tissues or from fat in the nerve lining. Because of the intraneural source of the image signal, the image provides a medically useful set of information about the internal state of the nerve such as the presence of irritation, nerve swelling (edema), compression, pinch or injury. Standard magnetic resonance images can show the outline of some nerves in portions of their courses but do not show the intrinsic signal from nerve water. Magnetic resonance neurography is used to evaluate major nerve compressions such as those affecting the sciatic nerve (e.g. piriformis syndrome), the brachial plexus nerves (e.g. thoracic outlet syndrome), the pudendal nerve, or virtually any named nerve in the body. A related technique for imaging neural tracts in the brain and spinal cord is called magnetic resonance tractography or diffusion tensor imaging.

Magnetic resonance imaging (MRI) is a medical imaging technique mostly used in radiology and nuclear medicine in order to investigate the anatomy and physiology of the body, and to detect pathologies including tumors, inflammation, neurological conditions such as stroke, disorders of muscles and joints, and abnormalities in the heart and blood vessels among others. Contrast agents may be injected intravenously or into a joint to enhance the image and facilitate diagnosis. Unlike CT and X-ray, MRI uses no ionizing radiation and is, therefore, a safe procedure suitable for diagnosis in children and repeated runs. Patients with specific non-ferromagnetic metal implants, cochlear implants, and cardiac pacemakers nowadays may also have an MRI in spite of effects of the strong magnetic fields. This does not apply on older devices, and details for medical professionals are provided by the device's manufacturer.
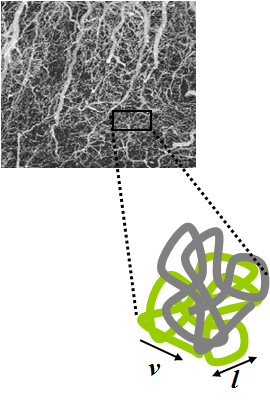
Intravoxel incoherent motion (IVIM) imaging is a concept and a method initially introduced and developed by Le Bihan et al. to quantitatively assess all the microscopic translational motions that could contribute to the signal acquired with diffusion MRI. In this model, biological tissue contains two distinct environments: molecular diffusion of water in the tissue, and microcirculation of blood in the capillary network (perfusion). The concept introduced by D. Le Bihan is that water flowing in capillaries mimics a random walk (Fig.1), as long as the assumption that all directions are represented in the capillaries is satisfied.

Perfusion MRI or perfusion-weighted imaging (PWI) is perfusion scanning by the use of a particular MRI sequence. The acquired data are then post-processed to obtain perfusion maps with different parameters, such as BV, BF, MTT and TTP.

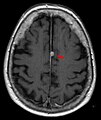
Multiple sclerosis (MS) can be pathologically defined as the presence of distributed glial scars (scleroses) in the central nervous system that must show dissemination in time (DIT) and in space (DIS) to be considered MS lesions.
Synthetic MRI is a simulation method in Magnetic Resonance Imaging (MRI), for generating contrast weighted images based on measurement of tissue properties. The synthetic (simulated) images are generated after an MR study, from parametric maps of tissue properties. It is thereby possible to generate several contrast weightings from the same acquisition. This is different from conventional MRI, where the signal acquired from the tissue is used to generate an image directly, often generating only one contrast weighting per acquisition. The synthetic images are similar in appearance to those normally acquired with an MRI scanner.
The history of magnetic resonance imaging (MRI) includes the work of many researchers who contributed to the discovery of nuclear magnetic resonance (NMR) and described the underlying physics of magnetic resonance imaging, starting early in the twentieth century. One researcher was American physicist Isidor Isaac Rabi who won the Nobel Prize in Physics in 1944 for his discovery of nuclear magnetic resonance, which is used in magnetic resonance imaging. MR imaging was invented by Paul C. Lauterbur who developed a mechanism to encode spatial information into an NMR signal using magnetic field gradients in September 1971; he published the theory behind it in March 1973.

An MRI pulse sequence in magnetic resonance imaging (MRI) is a particular setting of pulse sequences and pulsed field gradients, resulting in a particular image appearance.
Arterial spin labeling (ASL), also known as arterial spin tagging, is a magnetic resonance imaging technique used to quantify cerebral blood perfusion by labelling blood water as it flows throughout the brain. ASL specifically refers to magnetic labeling of arterial blood below or in the imaging slab, without the need of gadolinium contrast. A number of ASL schemes are possible, the simplest being flow alternating inversion recovery (FAIR) which requires two acquisitions of identical parameters with the exception of the out-of-slice saturation; the difference in the two images is theoretically only from inflowing spins, and may be considered a 'perfusion map'. The ASL technique was developed by John S. Leigh Jr, John A. Detre, Donald S. Williams, and Alan P. Koretsky in 1992.
Denis Le Bihan is a medical doctor, physicist, member of the Institut de France, member of the French Academy of Technologies and director since 2007 of NeuroSpin, an institution of the Atomic Energy and Alternative Energy Commission (CEA) in Saclay, dedicated to the study of the brain by magnetic resonance imaging (MRI) with a very high magnetic field. Denis Le Bihan has received international recognition for his outstanding work, introducing new imaging methods, particularly for the study of the human brain, as evidenced by the many international awards he has received, such as the Gold Medal of the International Society of Magnetic Resonance in Medicine (2001), the coveted Lounsbery Prize, the Louis D. Prize from the Institut de France, the prestigious Honda Prize (2012), the Louis-Jeantet Prize (2014), the Rhein Foundation Award (2021). His work has focused on the introduction, development and application of highly innovative methods, notably diffusion MRI.
Hyperpolarized 129Xe gas magnetic resonance imaging (MRI) is a medical imaging technique used to visualize the anatomy and physiology of body regions that are difficult to image with standard proton MRI. In particular, the lung, which lacks substantial density of protons, is particularly useful to be visualized with 129Xe gas MRI. This technique has promise as an early-detection technology for chronic lung diseases and imaging technique for processes and structures reliant on dissolved gases. 129Xe is a stable, naturally occurring isotope of xenon with 26.44% isotope abundance. It is one of two Xe isotopes, along with 131Xe, that has non-zero spin, which allows for magnetic resonance. 129Xe is used for MRI because its large electron cloud permits hyperpolarization and a wide range of chemical shifts. The hyperpolarization creates a large signal intensity, and the wide range of chemical shifts allows for identifying when the 129Xe associates with molecules like hemoglobin. 129Xe is preferred over 131Xe for MRI because 129Xe has spin 1/2, a longer T1, and 3.4 times larger gyromagnetic ratio (11.78 MHz/T).
Inversion recovery is a magnetic resonance imaging sequence that provides high contrast between tissue and lesion. It can be used to provide high T1 weighted image, high T2 weighted image, and to suppress the signals from fat, blood, or cerebrospinal fluid (CSF).
Magnetic resonance fingerprinting (MRF) is methodology in quantitative magnetic resonance imaging (MRI) characterized by a pseudo-randomized acquisition strategy. It involves creating unique signal patterns or 'fingerprints' for different materials or tissues after which a pattern recognition algorithm matches these fingerprints with a predefined dictionary of expected signal patterns. This process translates the data into quantitative maps, revealing information about the magnetic properties being investigated.