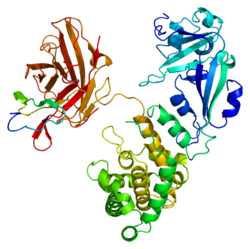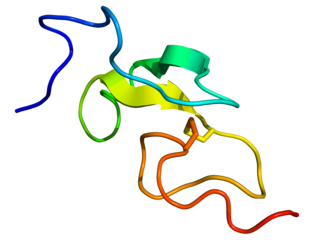
Epidermal growth factor (EGF) is a protein that stimulates cell growth and differentiation by binding to its receptor, EGFR. Human EGF is 6-kDa and has 53 amino acid residues and three intramolecular disulfide bonds.

Amphiregulin, also known as AREG, is a protein synthesized as a transmembrane glycoprotein with 252 aminoacids and it is encoded by the AREG gene. in humans.

Pleiotrophin (PTN) also known as heparin-binding brain mitogen (HBBM) or heparin-binding growth factor 8 (HBGF-8) or neurite growth-promoting factor 1 (NEGF1) or heparin affinity regulatory peptide (HARP) or heparin binding growth associated molecule (HB-GAM) is a protein that in humans is encoded by the PTN gene. Pleiotrophin is an 18-kDa growth factor that has a high affinity for heparin. It is structurally related to midkine and retinoic acid induced heparin-binding protein.
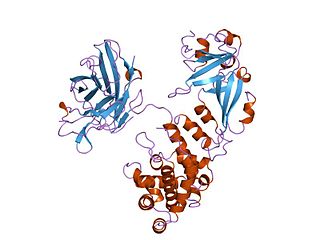
Diphtheria toxin is an exotoxin secreted mainly by Corynebacterium diphtheriae but also by Corynebacterium ulcerans and Corynebacterium pseudotuberculosis, the pathogenic bacterium that causes diphtheria. The toxin gene is encoded by a prophage called corynephage β. The toxin causes the disease in humans by gaining entry into the cell cytoplasm and inhibiting protein synthesis.

Midkine, also known as neurite growth-promoting factor 2 (NEGF2), is a protein that in humans is encoded by the MDK gene.

Sorting nexin-1 is a protein that in humans is encoded by the SNX1 gene. The protein encoded by this gene is a sorting nexin. SNX1 is a component of the retromer complex.

Growth factor receptor-bound protein 10 also known as insulin receptor-binding protein Grb-IR is a protein that in humans is encoded by the GRB10 gene.

Epiregulin (EPR) is a protein that in humans is encoded by the EREG gene.

Receptor tyrosine-protein kinase erbB-3, also known as HER3, is a membrane bound protein that in humans is encoded by the ERBB3 gene.

Receptor tyrosine-protein kinase erbB-4 is an enzyme that in humans is encoded by the ERBB4 gene. Alternatively spliced variants that encode different protein isoforms have been described; however, not all variants have been fully characterized.

Fibroblast growth factor receptor 4 (FGFR-4) is a protein that in humans is encoded by the FGFR4 gene. FGFR4 has also been designated as CD334.

BAG family molecular chaperone regulator 1 is a protein that in humans is encoded by the BAG1 gene.

The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in proteins: these repeats typically fold together to form a single, linear solenoid domain block as a functional unit.

Syndecan-3 is a protein that in humans is encoded by the SDC3 gene.

Teratocarcinoma-derived growth factor 1 is a protein that in humans is encoded by the TDGF1 gene. The protein is an extracellular, membrane-bound signaling protein that plays an essential role in embryonic development and tumor growth. Mutations in this gene are associated with forebrain defects. Pseudogenes of this gene are found on chromosomes 2, 3, 6, 8, 19 and X. Alternate splicing results in multiple transcript variants.

Growth factor receptor-bound protein 14 is a protein that in humans is encoded by the GRB14 gene.

Fibroblast growth factor-binding protein 1 is a protein that in humans is encoded by the FGFBP1 gene.

Stabilin-1 is a protein that in humans is encoded by the STAB1 gene.

Nardilysin is a protein that in humans is encoded by the NRD1 gene.
CRM197 is a non-toxic mutant of diphtheria toxin, currently used as a carrier protein for polysaccharides and haptens to make them immunogenic. There is some dispute about the toxicity of CRM197, with evidence that it is toxic to yeast cells and some mammalian cell lines.