
The insulin-like growth factors (IGFs) are proteins with high sequence similarity to insulin. IGFs are part of a complex system that cells use to communicate with their physiologic environment. This complex system consists of two cell-surface receptors, two ligands, a family of seven high-affinity IGF-binding proteins, as well as associated IGFBP degrading enzymes, referred to collectively as proteases.

Insulin-like growth factor 1 (IGF-1), also called somatomedin C, is a hormone similar in molecular structure to insulin which plays an important role in childhood growth, and has anabolic effects in adults.

Lipoprotein lipase (LPL) (EC 3.1.1.34, systematic name triacylglycerol acylhydrolase (lipoprotein-dependent)) is a member of the lipase gene family, which includes pancreatic lipase, hepatic lipase, and endothelial lipase. It is a water-soluble enzyme that hydrolyzes triglycerides in lipoproteins, such as those found in chylomicrons and very low-density lipoproteins (VLDL), into two free fatty acids and one monoacylglycerol molecule:
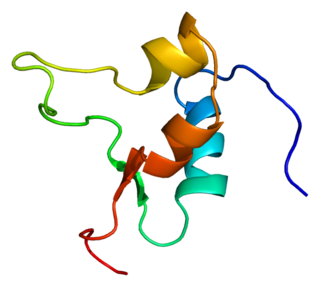
Insulin-like growth factor 2 (IGF-2) is one of three protein hormones that share structural similarity to insulin. The MeSH definition reads: "A well-characterized neutral peptide believed to be secreted by the liver and to circulate in the blood. It has growth-regulating, insulin-like and mitogenic activities. The growth factor has a major, but not absolute, dependence on somatotropin. It is believed to be a major fetal growth factor in contrast to insulin-like growth factor 1 (IGF-1), which is a major growth factor in adults."

The insulin-like growth factor 1 (IGF-1) receptor is a protein found on the surface of human cells. It is a transmembrane receptor that is activated by a hormone called insulin-like growth factor 1 (IGF-1) and by a related hormone called IGF-2. It belongs to the large class of tyrosine kinase receptors. This receptor mediates the effects of IGF-1, which is a polypeptide protein hormone similar in molecular structure to insulin. IGF-1 plays an important role in growth and continues to have anabolic effects in adults – meaning that it can induce hypertrophy of skeletal muscle and other target tissues. Mice lacking the IGF-1 receptor die late in development, and show a dramatic reduction in body mass. This testifies to the strong growth-promoting effect of this receptor.

The insulin-like growth factor-binding protein (IGFBP) serves as a transport protein for insulin-like growth factor 1 (IGF-1).

Insulin-like growth factor 2 receptor (IGF2R), also called the cation-independent mannose-6-phosphate receptor (CI-MPR) is a protein that in humans is encoded by the IGF2R gene. IGF2R is a multifunctional protein receptor that binds insulin-like growth factor 2 (IGF2) at the cell surface and mannose-6-phosphate (M6P)-tagged proteins in the trans-Golgi network.
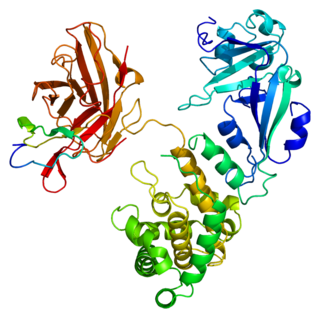
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of proteins that in humans is encoded by the HBEGF gene.

Insulin-like growth factor-binding protein 3, also known as IGFBP-3, is a protein that in humans is encoded by the IGFBP3 gene. IGFBP-3 is one of six IGF binding proteins that have highly conserved structures and bind the insulin-like growth factors IGF-1 and IGF-2 with high affinity. IGFBP-7, sometimes included in this family, shares neither the conserved structural features nor the high IGF affinity. Instead, IGFBP-7 binds IGF1R, which blocks IGF-1 and IGF-2 binding, resulting in apoptosis.
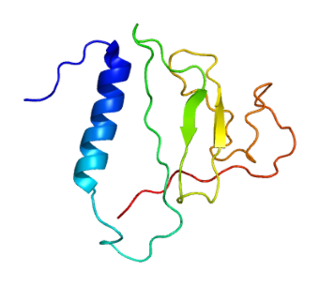
Insulin-like growth factor-binding protein 2 is a protein that in humans is encoded by the IGFBP2 gene.

Insulin-like growth factor-binding protein 5(IBF-5) is a protein that in humans is encoded by the IGFBP5 gene. An IGFBP5 gene was recently identified as being important for adaptation to varying water salinity in fish.

Insulin-like growth factor-binding protein 4 is a protein that in humans is encoded by the IGFBP4 gene.

Insulin-like growth factor-binding protein 6 (IGFBP-6) is a protein that in humans is encoded by the IGFBP6 gene.

Insulin-like growth factor-binding protein 1 (IBP-1) also known as placental protein 12 (PP12) is a protein that in humans is encoded by the IGFBP1 gene.

Insulin-like growth factor 2 mRNA-binding protein 1 is a protein that in humans is encoded by the IGF2BP1 gene.
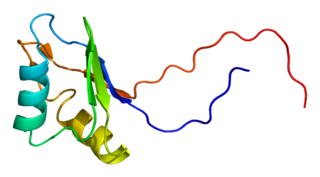
Insulin-like growth factor 2 mRNA-binding protein 2 is a protein that in humans is encoded by the IGF2BP2 gene.

ADP-ribosylation-like factor 6 interacting protein 4 (ARL6IP4), also called SRp25 is the product of the ARL6IP4 gene located on chromosome 12q24. 31. Its function is unknown.
Long arginine 3-IGF-1, abbreviated as IGF-1 LR3 or LR3-IGF-1, is a synthetic protein and lengthened analogue of human insulin-like growth factor 1 (IGF-1). It differs from native IGF-1 in that it possesses an arginine instead of a glutamic acid at the third position in its amino acid sequence, and also has an additional 13 amino acids at its N-terminus (MFPAMPLLSLFVN) ("long"), for a total of 83 amino acids. The consequences of these modifications are that IGF-1 LR3 retains the pharmacological activity of IGF-1 as an agonist of the IGF-1 receptor, has very low affinity for the insulin-like growth factor-binding proteins (IGFBPs), and has improved metabolic stability. As a result, it is approximately three times more potent than IGF-1, and possesses a significantly longer half-life of about 20–30 hours.
des(1-3)IGF-1 is a naturally occurring, endogenous protein, as well as drug, and truncated analogue of insulin-like growth factor 1 (IGF-1). des(1-3)IGF-1 lacks the first three amino acids at the N-terminus of IGF-1. As a result of this difference, it has considerably reduced binding to the insulin-like growth factor-binding proteins (IGFBPs) and enhanced potency relative to IGF-1.
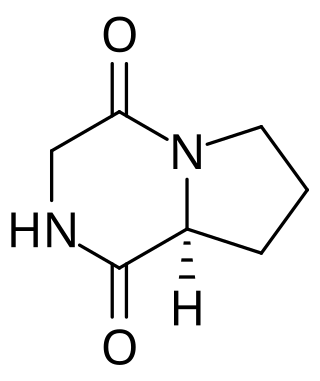
Cyclic glycine-proline (cGP) is a small neuroactive peptide that belongs to a group of bioactive 2,5-diketopiperazines (2,5-DKPs) and is also known as cyclo-glycine-proline. cGP is a neutral, stable naturally occurring compound and is endogenous to the human body; found in human plasma, breast milk and cerebrospinal fluid. DKPs are bioactive compounds often found in foods. Cyclic dipeptides such as 2,5 DKPs are formed by the cyclisation of two amino acids of linear peptides produced in heated or fermented foods. The bioactivity of cGP is a property of functional foods and presents in several matrices of foods including blackcurrants.























