Related Research Articles
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for regulating a variety of cellular processes.
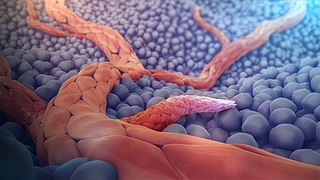
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

Colostrum, or first milk, is the first form of milk produced by the mammary glands of humans and other mammals immediately following delivery of the newborn. It may be called beestings when referring to the first milk of a cow or similar animal. Most species will begin to generate colostrum just prior to giving birth. Colostrum has an especially high amount of bioactive compounds compared to mature milk to give the newborn the best possible start to life. Specifically, colostrum contains antibodies to protect the newborn against disease and infection, and immune and growth factors and other bioactives that help to activate a newborn's immune system, jumpstart gut function, and seed a healthy gut microbiome in the first few days of life. The bioactives found in colostrum are essential for a newborn's health, growth and vitality. Colostrum strengthens a baby's immune system and is filled with white blood cells to protect it from infection.

Fibroblast growth factor 2, also known as basic fibroblast growth factor (bFGF) and FGF-β, is a growth factor and signaling protein encoded by the FGF2 gene. It binds to and exerts effects via specific fibroblast growth factor receptor (FGFR) proteins, themselves a family of closely related molecules. Fibroblast growth factor protein was first purified in 1975; soon thereafter three variants were isolated: 'basic FGF' (FGF2); Heparin-binding growth factor-2; and Endothelial cell growth factor-2. Gene sequencing revealed that this group is the same FGF2 protein and is a member of a family of FGF proteins.
Fibroblast growth factors (FGF) are a family of cell signalling proteins produced by macrophages; they are involved in a wide variety of processes, most notably as crucial elements for normal development in animal cells. Any irregularities in their function lead to a range of developmental defects. These growth factors typically act as systemic or locally circulating molecules of extracellular origin that activate cell surface receptors. A defining property of FGFs is that they bind to heparin and to heparan sulfate. Thus, some are sequestered in the extracellular matrix of tissues that contains heparan sulfate proteoglycans and are released locally upon injury or tissue remodeling.

Fibroblast growth factor 1, (FGF-1) also known as acidic fibroblast growth factor (aFGF), is a growth factor and signaling protein encoded by the FGF1 gene. It is synthesized as a 155 amino acid polypeptide, whose mature form is a non-glycosylated 17-18 kDa protein. Fibroblast growth factor protein was first purified in 1975, but soon afterwards others using different conditions isolated acidic FGF, Heparin-binding growth factor-1, and Endothelial cell growth factor-1. Gene sequencing revealed that this group was actually the same growth factor and that FGF1 was a member of a family of FGF proteins.

Fibroblast growth factor 23 (FGF23) is a protein and member of the fibroblast growth factor (FGF) family which participates in the regulation of phosphate in plasma and vitamin D metabolism. In humans it is encoded by the FGF23 gene. FGF23 decreases reabsorption of phosphate in the kidney. Mutations in FGF23 can lead to its increased activity, resulting in autosomal dominant hypophosphatemic rickets.
The fibroblast growth factor receptors (FGFR) are, as their name implies, receptors that bind to members of the fibroblast growth factor (FGF) family of proteins. Some of these receptors are involved in pathological conditions. For example, a point mutation in FGFR3 can lead to achondroplasia.

Fibroblast growth factor receptor 1 (FGFR1), also known as basic fibroblast growth factor receptor 1, fms-related tyrosine kinase-2 / Pfeiffer syndrome, and CD331, is a receptor tyrosine kinase whose ligands are specific members of the fibroblast growth factor family. FGFR1 has been shown to be associated with Pfeiffer syndrome, and clonal eosinophilias.

Keratinocyte growth factor is a protein that in humans is encoded by the FGF7 gene.

Fibroblast growth factor 10 is a protein that in humans is encoded by the FGF10 gene.

Fibroblast growth factor 8(FGF-8) is a protein that in humans is encoded by the FGF8 gene.

Fibroblast growth factor 5 is a protein that in humans is encoded by the FGF5 gene.

Fibroblast growth factor 18 (FGF18) is a protein that is encoded by the Fgf18 gene in humans. The protein was first discovered in 1998, when two newly-identified murine genes Fgf17 and Fgf18 were described and confirmed as being closely related by sequence homology to Fgf8. The three proteins were eventually grouped into the FGF8 subfamily, which contains several of the endocrine FGF superfamily members FGF8, FGF17, and FGF18. Subsequent studies identified FGF18's role in promoting chondrogenesis, and an apparent specific activity for the generation of the hyaline cartilage in articular joints.

Fibroblast growth factor 19 is a protein that in humans is encoded by the FGF19 gene. It functions as a hormone, regulating bile acid synthesis, with effects on glucose and lipid metabolism. Reduced synthesis, and blood levels, may be a factor in chronic bile acid diarrhea and in certain metabolic disorders.
Fibroblast growth factor 15 is a protein in mouse encoded by the Fgf15 gene. It is a member of the fibroblast growth factor (FGF) family but, like FGF19, FGF21 and FGF23, has endocrine functions. FGF19 is the orthologous protein in humans. They are often referred together as FGF15/19.
Sprifermin (INN), is a recombinant human fibroblast growth factor 18 (rhFGF18) analog, which is under development by TrialSpark for the treatment of osteoarthritis. FGF18 and sprifermin act via the Fibroblast Growth Factor Receptor (FGFR) family, with preferential activity via FGFR3c.
A bone growth factor is a growth factor that stimulates the growth of bone tissue.

Miproxifene phosphate is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group that was under development in Japan for the treatment of breast cancer but was abandoned and never marketed. It reached phase III clinical trials for this indication before development was discontinued. The drug is a phosphate ester and prodrug of miproxifene (DP-TAT-59) with improved water solubility that was better suited for clinical development. Miproxifene has been found to be 3- to 10-fold as potent as tamoxifen in inhibiting breast cancer cell growth in in vitro models. It is a derivative of afimoxifene (4-hydroxytamoxifen) in which an additional 4-isopropyl group is present in the β-phenyl ring.
Cenegermin, sold under the brand name Oxervate, also known as recombinant human nerve growth factor (rhNGF), is a recombinant form of human nerve growth factor (NGF). In July 2017, it was approved in the European Union as an eye drop formulation for the treatment of moderate or severe neurotrophic keratitis in adults.
References
- 1 2 Han SK (15 September 2015). "Growth Factor Therapy". Innovations and Advances in Wound Healing. Springer. pp. 206–. ISBN 978-3-662-46587-5.
- 1 2 "Trafermin - Kaken Pharmaceutical". AdisInsight. Springer Nature Switzerland AG.
- ↑ "Trafermin". Drugs.com.
- ↑ Kitamura M, Akamatsu M, Kawanami M, Furuichi Y, Fujii T, Mori M, et al. (April 2016). "Randomized Placebo-Controlled and Controlled Non-Inferiority Phase III Trials Comparing Trafermin, a Recombinant Human Fibroblast Growth Factor 2, and Enamel Matrix Derivative in Periodontal Regeneration in Intrabony Defects". Journal of Bone and Mineral Research. 31 (4): 806–814. doi: 10.1002/jbmr.2738 . PMID 26547659.
- ↑ Barnard JA, McHugh KM (10 May 2006). "Growth Factors in the Gastrointestinal Tract". In Barrett KE, Ghishan FK, Merchant JL, Said HM, Wood JD (eds.). Physiology of the Gastrointestinal Tract. Academic Press. pp. 216–. ISBN 978-0-08-045615-7.
- ↑ Hamid M. Said (4 July 2012). Physiology of the Gastrointestinal Tract, Two Volume Set. Academic Press. pp. 235–. ISBN 978-0-12-382027-3.