
Within a nervous system, a neuron, neurone, or nerve cell is an electrically excitable cell that fires electric signals called action potentials across a neural network. Neurons communicate with other cells via synapses, which are specialized connections that commonly use minute amounts of chemical neurotransmitters to pass the electric signal from the presynaptic neuron to the target cell through the synaptic gap.
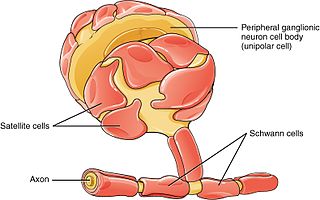
Schwann cells or neurolemmocytes are the principal glia of the peripheral nervous system (PNS). Glial cells function to support neurons and in the PNS, also include satellite cells, olfactory ensheathing cells, enteric glia and glia that reside at sensory nerve endings, such as the Pacinian corpuscle. The two types of Schwann cells are myelinating and nonmyelinating. Myelinating Schwann cells wrap around axons of motor and sensory neurons to form the myelin sheath. The Schwann cell promoter is present in the downstream region of the human dystrophin gene that gives shortened transcript that are again synthesized in a tissue-specific manner.

In neuroscience, long-term potentiation (LTP) is a persistent strengthening of synapses based on recent patterns of activity. These are patterns of synaptic activity that produce a long-lasting increase in signal transmission between two neurons. The opposite of LTP is long-term depression, which produces a long-lasting decrease in synaptic strength.
An inhibitory postsynaptic potential (IPSP) is a kind of synaptic potential that makes a postsynaptic neuron less likely to generate an action potential. IPSPs were first investigated in motorneurons by David P. C. Lloyd, John Eccles and Rodolfo Llinás in the 1950s and 1960s. The opposite of an inhibitory postsynaptic potential is an excitatory postsynaptic potential (EPSP), which is a synaptic potential that makes a postsynaptic neuron more likely to generate an action potential. IPSPs can take place at all chemical synapses, which use the secretion of neurotransmitters to create cell to cell signalling. Inhibitory presynaptic neurons release neurotransmitters that then bind to the postsynaptic receptors; this induces a change in the permeability of the postsynaptic neuronal membrane to particular ions. An electric current that changes the postsynaptic membrane potential to create a more negative postsynaptic potential is generated, i.e. the postsynaptic membrane potential becomes more negative than the resting membrane potential, and this is called hyperpolarisation. To generate an action potential, the postsynaptic membrane must depolarize—the membrane potential must reach a voltage threshold more positive than the resting membrane potential. Therefore, hyperpolarisation of the postsynaptic membrane makes it less likely for depolarisation to sufficiently occur to generate an action potential in the postsynaptic neurone.
In neurophysiology, long-term depression (LTD) is an activity-dependent reduction in the efficacy of neuronal synapses lasting hours or longer following a long patterned stimulus. LTD occurs in many areas of the CNS with varying mechanisms depending upon brain region and developmental progress.

An excitatory synapse is a synapse in which an action potential in a presynaptic neuron increases the probability of an action potential occurring in a postsynaptic cell. Neurons form networks through which nerve impulses travels, each neuron often making numerous connections with other cells of neurons. These electrical signals may be excitatory or inhibitory, and, if the total of excitatory influences exceeds that of the inhibitory influences, the neuron will generate a new action potential at its axon hillock, thus transmitting the information to yet another cell.

Brain-derived neurotrophic factor (BDNF), or abrineurin, is a protein that, in humans, is encoded by the BDNF gene. BDNF is a member of the neurotrophin family of growth factors, which are related to the canonical nerve growth factor (NGF), a family which also includes NT-3 and NT-4/NT-5. Neurotrophic factors are found in the brain and the periphery. BDNF was first isolated from a pig brain in 1982 by Yves-Alain Barde and Hans Thoenen.
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person's lifespan, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis. Synaptogenesis is particularly important during an individual's critical period, during which there is a certain degree of synaptic pruning due to competition for neural growth factors by neurons and synapses. Processes that are not used, or inhibited during their critical period will fail to develop normally later on in life.
Molecular neuroscience is a branch of neuroscience that observes concepts in molecular biology applied to the nervous systems of animals. The scope of this subject covers topics such as molecular neuroanatomy, mechanisms of molecular signaling in the nervous system, the effects of genetics and epigenetics on neuronal development, and the molecular basis for neuroplasticity and neurodegenerative diseases. As with molecular biology, molecular neuroscience is a relatively new field that is considerably dynamic.

Neuregulin 1, or NRG1, is a gene of the epidermal growth factor family that in humans is encoded by the NRG1 gene. NRG1 is one of four proteins in the neuregulin family that act on the EGFR family of receptors. Neuregulin 1 is produced in numerous isoforms by alternative splicing, which allows it to perform a wide variety of functions. It is essential for the normal development of the nervous system and the heart.

Neurexins (NRXN) are a family of presynaptic cell adhesion proteins that have roles in connecting neurons at the synapse. They are located mostly on the presynaptic membrane and contain a single transmembrane domain. The extracellular domain interacts with proteins in the synaptic cleft, most notably neuroligin, while the intracellular cytoplasmic portion interacts with proteins associated with exocytosis. Neurexin and neuroligin "shake hands," resulting in the connection between the two neurons and the production of a synapse. Neurexins mediate signaling across the synapse, and influence the properties of neural networks by synapse specificity. Neurexins were discovered as receptors for α-latrotoxin, a vertebrate-specific toxin in black widow spider venom that binds to presynaptic receptors and induces massive neurotransmitter release. In humans, alterations in genes encoding neurexins are implicated in autism and other cognitive diseases, such as Tourette syndrome and schizophrenia.

Receptor tyrosine-protein kinase erbB-4 is an enzyme that in humans is encoded by the ERBB4 gene. Alternatively spliced variants that encode different protein isoforms have been described; however, not all variants have been fully characterized.

Neuregulin 2, also known as NRG2, is a protein which in humans is encoded by the NRG2 gene.
The glutamate hypothesis of schizophrenia models the subset of pathologic mechanisms of schizophrenia linked to glutamatergic signaling. The hypothesis was initially based on a set of clinical, neuropathological, and, later, genetic findings pointing at a hypofunction of glutamatergic signaling via NMDA receptors. While thought to be more proximal to the root causes of schizophrenia, it does not negate the dopamine hypothesis, and the two may be ultimately brought together by circuit-based models. The development of the hypothesis allowed for the integration of the GABAergic and oscillatory abnormalities into the converging disease model and made it possible to discover the causes of some disruptions.

Neuregulin 3, also known as NRG3, is a neural-enriched member of the neuregulin protein family which in humans is encoded by the NRG3 gene. The NRGs are a group of signaling proteins part of the superfamily of epidermal growth factor, EGF like polypeptide growth factor. These groups of proteins possess an 'EGF-like domain' that consists of six cysteine residues and three disulfide bridges predicted by the consensus sequence of the cysteine residues.

Neuregulin 4 also known as NRG4 is a member of the neuregulin protein family which in humans is encoded by the NRG4 gene.
The spine apparatus (SA) is a specialized form of endoplasmic reticulum (ER) that is found in a subpopulation of dendritic spines in central neurons. It was discovered by Edward George Gray in 1959 when he applied electron microscopy to fixed cortical tissue. The SA consists of a series of stacked discs that are connected to each other and to the dendritic system of ER-tubules. The actin binding protein synaptopodin is an essential component of the SA. Mice that lack the gene for synaptopodin do not form a spine apparatus. The SA is believed to play a role in synaptic plasticity, learning and memory, but the exact function of the spine apparatus is still enigmatic.
Activity-dependent plasticity is a form of functional and structural neuroplasticity that arises from the use of cognitive functions and personal experience; hence, it is the biological basis for learning and the formation of new memories. Activity-dependent plasticity is a form of neuroplasticity that arises from intrinsic or endogenous activity, as opposed to forms of neuroplasticity that arise from extrinsic or exogenous factors, such as electrical brain stimulation- or drug-induced neuroplasticity. The brain's ability to remodel itself forms the basis of the brain's capacity to retain memories, improve motor function, and enhance comprehension and speech amongst other things. It is this trait to retain and form memories that is associated with neural plasticity and therefore many of the functions individuals perform on a daily basis. This plasticity occurs as a result of changes in gene expression which are triggered by signaling cascades that are activated by various signaling molecules during increased neuronal activity.

Gerald D. Fischbach is an American neuroscientist. He received his M.D. from the Weill Cornell Medical College of Cornell University in 1965 before beginning his research career at the National Institutes of Health in 1966, where his research focused on the mechanisms of neuromuscular junctions. After his tenure at the National Institutes of Health, Fischbach was a professor at Harvard University Medical School from 1972 to 1981 and from 1990 to 1998 and the Washington University School of Medicine from 1981 to 1990. In 1998, he was named the director of the National Institute of Neurological Disorders and Stroke before becoming the Vice President and Dean of the Health and Biomedical Sciences, the Dean of the Faculty of Medicine, and the Dean of the Faculty of Health Sciences at Columbia University from 2001 to 2006. Gerald Fischbach currently serves as the scientific director overseeing the Simons Foundation Autism Research Initiative. Throughout Fischbach's career, much of his research has focused on the formation and function of the neuromuscular junction, which stemmed from his innovative use of cell culture to study synaptic mechanisms.

Research into the mental disorder of schizophrenia, involves multiple animal models as a tool, including in the preclinical stage of drug development.













