 | |
| Clinical data | |
|---|---|
| Trade names | Forteo, Forsteo |
| Biosimilars | Bonsity, [1] Kauliv, [2] Livogiva, [3] Osnuvo, [4] Qutavina, [5] Sondelbay, [6] Teribone, [7] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603018 |
| License data |
|
| Pregnancy category | |
| Routes of administration | Subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Metabolism | Liver (nonspecific proteolysis) |
| Elimination half-life | Subcutaneous: 1 hour |
| Excretion | Kidney (metabolites) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.168.733 |
| Chemical and physical data | |
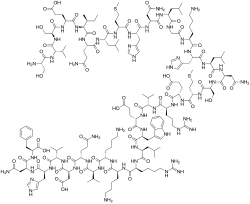
| Formula | C181H291N55O51S2 |
| Molar mass | 4117.77 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Teriparatide, sold under the brand name Forteo, is a form of parathyroid hormone (PTH) consisting of the first (N-terminus) 34 amino acids, which is the portion of the hormone activating the Parathyroid hormone 1 receptor. [12] It is an effective anabolic (promoting bone formation) agent [14] used in the treatment of some forms of osteoporosis. [12] [15] Teriparatide is a recombinant human parathyroid hormone analog (PTH 1-34). [12] It has an identical sequence to the 34 N-terminal amino acids of the 84-amino acid human parathyroid hormone. [12]
