
Escherichia coli O157:H7 is a serotype of the bacterial species Escherichia coli and is one of the Shiga-like toxin–producing types of E. coli. It is a cause of disease, typically foodborne illness, through consumption of contaminated and raw food, including raw milk and undercooked ground beef. Infection with this type of pathogenic bacteria may lead to hemorrhagic diarrhea, and to kidney failure; these have been reported to cause the deaths of children younger than five years of age, of elderly patients, and of patients whose immune systems are otherwise compromised.

Shiga toxins are a family of related toxins with two major groups, Stx1 and Stx2, expressed by genes considered to be part of the genome of lambdoid prophages. The toxins are named after Kiyoshi Shiga, who first described the bacterial origin of dysentery caused by Shigella dysenteriae. Shiga-like toxin (SLT) is a historical term for similar or identical toxins produced by Escherichia coli. The most common sources for Shiga toxin are the bacteria S. dysenteriae and some serotypes of Escherichia coli (STEC), which includes serotypes O157:H7, and O104:H4.

Hemolytic–uremic syndrome (HUS) is a group of blood disorders characterized by low red blood cells, acute kidney failure, and low platelets. Initial symptoms typically include bloody diarrhea, fever, vomiting, and weakness. Kidney problems and low platelets then occur as the diarrhea progresses. Children are more commonly affected, but most children recover without permanent damage to their health, although some children may have serious and sometimes life-threatening complications. Adults, especially the elderly, may present a more complicated presentation. Complications may include neurological problems and heart failure.
Humanized antibodies are antibodies from non-human species whose protein sequences have been modified to increase their similarity to antibody variants produced naturally in humans. The process of "humanization" is usually applied to monoclonal antibodies developed for administration to humans. Humanization can be necessary when the process of developing a specific antibody involves generation in a non-human immune system. The protein sequences of antibodies produced in this way are partially distinct from homologous antibodies occurring naturally in humans, and are therefore potentially immunogenic when administered to human patients. The International Nonproprietary Names of humanized antibodies end in -zumab, as in omalizumab.
The nomenclature of monoclonal antibodies is a naming scheme for assigning generic, or nonproprietary, names to monoclonal antibodies. An antibody is a protein that is produced in B cells and used by the immune system of humans and other vertebrate animals to identify a specific foreign object like a bacterium or a virus. Monoclonal antibodies are those that were produced in identical cells, often artificially, and so share the same target object. They have a wide range of applications including medical uses.
The AB5 toxins are six-component protein complexes secreted by certain pathogenic bacteria known to cause human diseases such as cholera, dysentery, and hemolytic–uremic syndrome. One component is known as the A subunit, and the remaining five components are B subunits. All of these toxins share a similar structure and mechanism for entering targeted host cells. The B subunit is responsible for binding to receptors to open up a pathway for the A subunit to enter the cell. The A subunit is then able to use its catalytic machinery to take over the host cell's regular functions.
Escherichia coli O121 is a pathogenic serotype of Escherichia coli, associated with Shiga toxin, intestinal bleeding, and hemolytic-uremic syndrome (HUS). HUS, if left untreated, can lead to kidney failure.
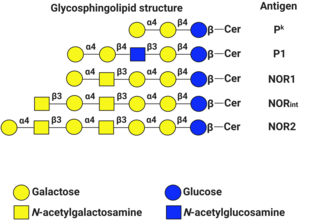
P1PK is a human blood group system based upon the A4GALT gene on chromosome 22. The P antigen was first described by Karl Landsteiner and Philip Levine in 1927. The P1PK blood group system consists of three glycosphingolipid antigens: Pk, P1 and NOR. In addition to glycosphingolipids, terminal Galα1→4Galβ structures are present on complex-type N-glycans. The GLOB antigen is now the member of the separate GLOB blood group system.

Escherichia coli O104:H21 is a rare serotype of Escherichia coli, a species of bacteria that lives in the lower intestines of mammals. Although there are many serotypes of E. coli, when in animals, there are benefits or do not cause disease. Some serotypes of E. coli have been recognized as pathogenic to humans, e.g. E. coli O157:H7, E. coli O121 and E. coli O104:H21.
Dacetuzumab is a humanized monoclonal antibody being developed for the treatment of CD40-positive cancers like non-Hodgkin's lymphoma and hematological malignancies.

A ribosome-inactivating protein (RIP) is a protein synthesis inhibitor that acts at the eukaryotic ribosome. This protein family describes a large family of such proteins that work by acting as rRNA N-glycosylase. They inactivate 60S ribosomal subunits by an N-glycosidic cleavage, which releases a specific adenine base from the sugar-phosphate backbone of 28S rRNA. RIPs exist in bacteria and plants.
InDevR is a biotechnology company in Boulder, Colorado, that develops advanced life science instrumentation and assays for analysis of viruses and other microorganisms, as well as protein detection and characterization, with product focus on Virus Quantification and pathogen detection/identification.

A novel strain of Escherichia coli O104:H4 bacteria caused a serious outbreak of foodborne illness focused in northern Germany in May through June 2011. The illness was characterized by bloody diarrhea, with a high frequency of serious complications, including hemolytic–uremic syndrome (HUS), a condition that requires urgent treatment. The outbreak was originally thought to have been caused by an enterohemorrhagic (EHEC) strain of E. coli, but it was later shown to have been caused by an enteroaggregative E. coli (EAEC) strain that had acquired the genes to produce Shiga toxins, present in organic fenugreek sprouts.
Escherichia coli O104:H4 is an enteroaggregative Escherichia coli strain of the bacterium Escherichia coli, and the cause of the 2011 Escherichia coli O104:H4 outbreak. The "O" in the serological classification identifies the cell wall lipopolysaccharide antigen, and the "H" identifies the flagella antigen.
Shigatoxigenic Escherichia coli (STEC) and verotoxigenic E. coli (VTEC) are strains of the bacterium Escherichia coli that produce Shiga toxin. Only a minority of the strains cause illness in humans. The ones that do are collectively known as enterohemorrhagic E. coli (EHEC) and are major causes of foodborne illness. When infecting the large intestine of humans, they often cause gastroenteritis, enterocolitis, and bloody diarrhea and sometimes cause a severe complication called hemolytic-uremic syndrome (HUS). Cattle is an important natural reservoir for EHEC because the colonised adult ruminants are asymptomatic. This is because they lack vascular expression of the target receptor for Shiga toxins. The group and its subgroups are known by various names. They are distinguished from other strains of intestinal pathogenic E. coli including enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), enteroinvasive E. coli (EIEC), enteroaggregative E. coli (EAEC), and diffusely adherent E. coli (DAEC).
Antimotility agents are drugs used to alleviate the symptoms of diarrhea. These include loperamide (Imodium), diphenoxylate with atropine (Lomotil), and opiates such as paregoric, tincture of opium, codeine, and morphine. In diarrhea caused by invasive pathogens such as Salmonella, Shigella, and Campylobacter, the use of such agents has generally been strongly discouraged, though evidence is lacking that they are harmful when administered in combination with antibiotics in Clostridium difficile cases. Use of antimotility agents in children and the elderly has also been discouraged in treatment of EHEC due to an increased rate of hemolytic uremic syndrome.

Escherichia coli is a gram-negative, rod-shaped bacterium that is commonly found in the lower intestine of warm-blooded organisms (endotherms). Most E. coli strains are harmless, but pathogenic varieties cause serious food poisoning, septic shock, meningitis, or urinary tract infections in humans. Unlike normal flora E. coli, the pathogenic varieties produce toxins and other virulence factors that enable them to reside in parts of the body normally not inhabited by E. coli, and to damage host cells. These pathogenic traits are encoded by virulence genes carried only by the pathogens.
Enteroaggregative Escherichia coli are a pathotype of Escherichia coli which cause acute and chronic diarrhea in both the developed and developing world. They may also cause urinary tract infections. EAEC are defined by their "stacked-brick" pattern of adhesion to the human laryngeal epithelial cell line HEp-2. The pathogenesis of EAEC involves the aggregation of and adherence of the bacteria to the intestinal mucosa, where they elaborate enterotoxins and cytotoxins that damage host cells and induce inflammation that results in diarrhea.
Antivirulence is the concept of blocking virulence factors. In regards to bacteria, the idea is to design agents that block virulence rather than kill bacteria en masse, as the current regime results in much more selective pressure.
In the medical field of immunology, nanoCLAMP affinity reagents are recombinant 15 kD antibody mimetic proteins selected for tight, selective and gently reversible binding to target molecules. The nanoCLAMP scaffold is based on an IgG-like, thermostable carbohydrate binding module family 32 (CBM32) from a Clostridium perfringens hyaluronidase. The shape of nanoCLAMPs approximates a cylinder of approximately 4 nm in length and 2.5 nm in diameter, roughly the same size as a nanobody. nanoCLAMPs to specific targets are generated by varying the amino acid sequences and sometimes the length of three solvent exposed, adjacent loops that connect the beta strands making up the beta-sandwich fold, conferring binding affinity and specificity for the target.





