
Interferons are a group of signaling proteins made and released by host cells in response to the presence of several viruses. In a typical scenario, a virus-infected cell will release interferons causing nearby cells to heighten their anti-viral defenses.

Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is acute if it resolves within six months, and chronic if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer.

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.
Hepatitis D is a type of viral hepatitis caused by the hepatitis delta virus (HDV). HDV is one of five known hepatitis viruses: A, B, C, D, and E. HDV is considered to be a satellite because it can propagate only in the presence of the hepatitis B virus (HBV). Transmission of HDV can occur either via simultaneous infection with HBV (coinfection) or superimposed on chronic hepatitis B or hepatitis B carrier state (superinfection).

Hepatitis A is an infectious disease of the liver caused by Hepatovirus A (HAV); it is a type of viral hepatitis. Many cases have few or no symptoms, especially in the young. The time between infection and symptoms, in those who develop them, is 2–6 weeks. When symptoms occur, they typically last 8 weeks and may include nausea, vomiting, diarrhea, jaundice, fever, and abdominal pain. Around 10–15% of people experience a recurrence of symptoms during the 6 months after the initial infection. Acute liver failure may rarely occur, with this being more common in the elderly.

Hepatitis E is inflammation of the liver caused by infection with the hepatitis E virus (HEV); it is a type of viral hepatitis. Hepatitis E has mainly a fecal-oral transmission route that is similar to hepatitis A, although the viruses are unrelated. In retrospect, the earliest known epidemic of hepatitis E occurred in 1955 in New Delhi, but the virus was not isolated until 1983 by Russian scientists investigating an outbreak in Afghanistan. HEV is a positive-sense, single-stranded, nonenveloped, RNA icosahedral virus and one of five known human hepatitis viruses: A, B, C, D, and E.

Hepadnaviridae is a family of viruses. Humans, apes, and birds serve as natural hosts. There are currently 18 species in this family, divided among 5 genera. Its best-known member is hepatitis B virus. Diseases associated with this family include: liver infections, such as hepatitis, hepatocellular carcinomas, and cirrhosis. It is the sole accepted family in the order Blubervirales.
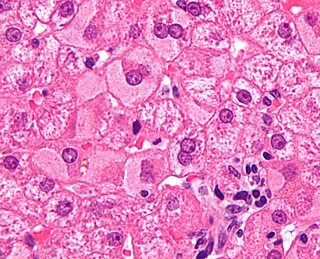
Viral hepatitis is liver inflammation due to a viral infection. It may present in acute form as a recent infection with relatively rapid onset, or in chronic form, typically progressing from a long-lasting asymptomatic condition up to a decompensated hepatic disease and hepatocellular carcinoma (HCC).

Rituximab, sold under the brand name Rituxan among others, is a monoclonal antibody medication used to treat certain autoimmune diseases and types of cancer. It is used for non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis, idiopathic thrombocytopenic purpura, pemphigus vulgaris, myasthenia gravis and Epstein–Barr virus-positive mucocutaneous ulcers. It is given by slow intravenous infusion. Biosimilars of Rituxan include Blitzima, Riabni, Ritemvia, Rituenza, Rixathon, Ruxience, and Truxima.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.
Bavituximab (PGN401) is a human-mouse chimeric monoclonal antibody against phosphatidylserine, which is a component of cell membranes that is exposed when a cell is transformed into solid tumor cancer cell or dies, and when cells are infected with hepatitis C. The process of cell death is highly controlled and so there usually no immune response to phosphatidylserine but when bavituximab binds to it, the conjugate appears to stimulate an immune response in humans.
Exbivirumab is a human monoclonal antibody developed for the treatment of hepatitis B infections.
Tuvirumab is a human monoclonal antibody for the treatment of patients with chronic hepatitis B. It has undergone Phase I clinical trials in 2001.
Ofatumumab is a fully human monoclonal antibody to CD20, which appears to provide rapid B-cell depletion. Under the brand name Kesimpta, it is approved for the treatment of multiple sclerosis in the United States as well as in the European Union and other regions. Under the brand name Arzerra, it is approved for the treatment of certain types of chronic lymphocytic leukemia (CLL) in the United States. It is sold by Novartis under license from Genmab.

CD81 molecule, also known as CD81, is a protein which in humans is encoded by the CD81 gene. It is also known as 26 kDa cell surface protein, TAPA-1, and Tetraspanin-28 (Tspan-28).

Hepatitis B is an infectious disease caused by the Hepatitis B virus (HBV) that affects the liver; it is a type of viral hepatitis. It can cause both acute and chronic infection.

Hepatitis B virus (HBV) is a partially double-stranded DNA virus, a species of the genus Orthohepadnavirus and a member of the Hepadnaviridae family of viruses. This virus causes the disease hepatitis B.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy.

Sir Michael Houghton is a British scientist and Nobel Prize laureate. Along with Qui-Lim Choo, George Kuo and Daniel W. Bradley, he co-discovered Hepatitis C in 1989. He also co-discovered the Hepatitis D genome in 1986. The discovery of the Hepatitis C virus (HCV) led to the rapid development of diagnostic reagents to detect HCV in blood supplies, which has reduced the risk of acquiring HCV through blood transfusion from one in three to about one in two million. It is estimated that antibody testing has prevented at least 40,000 new infections per year in the US alone and many more worldwide.
Lenvervimab is a monoclonal antibody that is being investigated for hepatitis B.











