The cluster of differentiation is a protocol used for the identification and investigation of cell surface molecules providing targets for immunophenotyping of cells. In terms of physiology, CD molecules can act in numerous ways, often acting as receptors or ligands important to the cell. A signal cascade is usually initiated, altering the behavior of the cell. Some CD proteins do not play a role in cell signaling, but have other functions, such as cell adhesion. CD for humans is numbered up to 371.

A monoclonal antibody is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
In immunology, antiserum is a blood serum containing antibodies that is used to spread passive immunity to many diseases via blood donation (plasmapheresis). For example, convalescent serum, passive antibody transfusion from a previous human survivor, used to be the only known effective treatment for ebola infection with a high success rate of 7 out of 8 patients surviving.
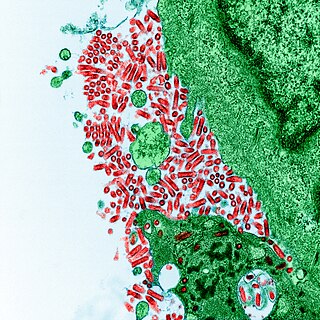
Rabies virus, scientific name Rabies lyssavirus, is a neurotropic virus that causes rabies in animals, including humans. Rabies transmission can occur through the saliva of animals and less commonly through contact with human saliva. Rabies lyssavirus, like many rhabdoviruses, has an extremely wide host range. In the wild it has been found infecting many mammalian species, while in the laboratory it has been found that birds can be infected, as well as cell cultures from mammals, birds, reptiles and insects. Rabies is reported in more than 150 countries and on all continents except Antarctica. The main burden of disease is reported in Asia and Africa, but some cases have been reported also in Europe in the past 10 years, especially in returning travellers.
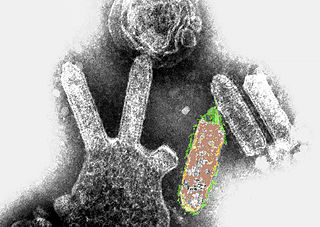
Australian bat lyssavirus (ABLV), originally named Pteropid lyssavirus (PLV), is a enzootic virus closely related to the rabies virus. It was first identified in a 5-month-old juvenile black flying fox collected near Ballina in northern New South Wales, Australia, in January 1995 during a national surveillance program for the recently identified Hendra virus. ABLV is the seventh member of the genus Lyssavirus and the only Lyssavirus member present in Australia. ABLV has been categorized to the Phylogroup I of the Lyssaviruses.

Antibody-dependent cellular cytotoxicity (ADCC), also referred to as antibody-dependent cell-mediated cytotoxicity, is a mechanism of cell-mediated immune defense whereby an effector cell of the immune system kills a target cell, whose membrane-surface antigens have been bound by specific antibodies. It is one of the mechanisms through which antibodies, as part of the humoral immune response, can act to limit and contain infection.
Entry inhibitors, also known as fusion inhibitors, are a class of antiviral drugs that prevent a virus from entering a cell, for example, by blocking a receptor. Entry inhibitors are used to treat conditions such as HIV and hepatitis D.
Palivizumab, sold under the brand name Synagis, is a monoclonal antibody produced by recombinant DNA technology used to prevent severe disease caused by respiratory syncytial virus (RSV) infections. It is recommended for infants at high-risk for RSV due to conditions such as prematurity or other medical problems including heart or lung diseases.

Antonio Lanzavecchia is an Italian and Swiss immunologist. As a fellow of Collegio Borromeo he obtained a degree with honors in Medicine in 1976 from the University of Pavia where he specialized in Pediatrics and Infectious Diseases. He is Head Human Immunology Program, Istituto Nazionale di Genetica Molecolare-INGM, Milan and SVP Senior research Fellow, Humabs/Vir Biotechnology, Bellinzona and San Francisco (USA). Since 2017, he is also Professor at the Faculty of Biomedical Sciences of the Università della Svizzera italiana (USI).
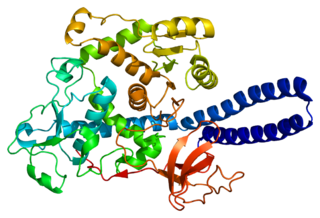
Alpha-(1,6)-fucosyltransferase is an enzyme that in humans is encoded by the FUT8 gene.
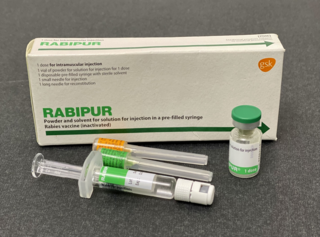
The rabies vaccine is a vaccine used to prevent rabies. There are several rabies vaccines available that are both safe and effective. Vaccinations must be administered prior to rabies virus exposure or within the latent period after exposure to prevent the disease. Transmission of rabies virus to humans typically occurs through a bite or scratch from an infectious animal, but exposure can occur through indirect contact with the saliva from an infectious individual.

Rabies is a viral disease that causes encephalitis in humans and other mammals. It was historically referred to as hydrophobia due to the symptom of panic when presented with liquids to drink. Early symptoms can include fever and abnormal sensations at the site of exposure. These symptoms are followed by one or more of the following symptoms: nausea, vomiting, violent movements, uncontrolled excitement, fear of water, an inability to move parts of the body, confusion, and loss of consciousness. Once symptoms appear, the result is virtually always death, regardless of treatment. The time period between contracting the disease and the start of symptoms is usually one to three months but can vary from less than one week to more than one year. The time depends on the distance the virus must travel along peripheral nerves to reach the central nervous system.
Regavirumab is a human monoclonal antibody against infections with cytomegalovirus.
Siltuximab is a chimeric monoclonal antibody. It binds to interleukin-6. Siltuximab has been investigated for the treatment of neoplastic diseases: metastatic renal cell cancer, prostate cancer, other types of cancer, and for Castleman's disease.
CR6261 is a monoclonal antibody that binds to a broad range of the influenza virus including the 1918 "Spanish flu" (SC1918/H1) and to a virus of the H5N1 class of avian influenza that jumped from chickens to a human in Vietnam in 2004 (Viet04/H5). In contrast to most antibodies generated by exposure to influenza, which can only neutralize a few strains from within a single virus subtype, CR6261 neutralizes numerous strains from multiple subtypes. CR6261 recognizes a highly conserved helical region in the membrane-proximal stem of hemagglutinin, the predominant protein on the surface of the influenza virus. Based upon the conservation of the amino acid sequence on this part of hemagglutinin, CR6261 is predicted to neutralize roughly 50% of all flu viruses. It was found by The Scripps Research Institute and the Dutch biopharmaceutical company, Crucell.
A neutralizing antibody (NAb) is an antibody that defends a cell from a pathogen or infectious particle by neutralizing any effect it has biologically. Neutralization renders the particle no longer infectious or pathogenic. Neutralizing antibodies are part of the humoral response of the adaptive immune system against viruses, intracellular bacteria and microbial toxin. By binding specifically to surface structures (antigen) on an infectious particle, neutralizing antibodies prevent the particle from interacting with its host cells it might infect and destroy.

ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease. Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Gary Kobinger, a research scientist at the NML and underwent further development under license by Mapp Biopharmaceutical. ZMapp was first used on humans during the Western African Ebola virus epidemic, having only been previously tested on animals and not yet subjected to a randomized controlled trial. The National Institutes of Health (NIH) ran a clinical trial starting in January 2015 with subjects from Sierra Leone, Guinea, and Liberia aiming to enroll 200 people, but the epidemic waned and the trial closed early, leaving it too statistically underpowered to give a meaningful result about whether ZMapp worked.
Ansuvimab, sold under the brand name Ebanga, is a monoclonal antibody medication for the treatment of Zaire ebolavirus (Ebolavirus) infection.
Human betaherpesvirus 6A (HHV-6A) is a species of virus in the genus Roseolovirus, subfamily Betaherpesvirinae, family Herpesviridae, and order Herpesvirales.
Bamlanivimab is a monoclonal antibody developed by AbCellera Biologics and Eli Lilly as a treatment for COVID-19. The medication was granted an emergency use authorization (EUA) by the US Food and Drug Administration (FDA) in November 2020, and the EUA was revoked in April 2021.







