
Sepsis, or blood poisoning, is a life-threatening condition that arises when the body's response to infection causes injury to its own tissues and organs.

Septic shock is a potentially fatal medical condition that occurs when sepsis, which is organ injury or damage in response to infection, leads to dangerously low blood pressure and abnormalities in cellular metabolism. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) defines septic shock as a subset of sepsis in which particularly profound circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. Patients with septic shock can be clinically identified by requiring a vasopressor to maintain a mean arterial pressure of 65 mm Hg or greater and having serum lactate level greater than 2 mmol/L (>18 mg/dL) in the absence of hypovolemia. This combination is associated with hospital mortality rates greater than 40%.

Procalcitonin (PCT) is a peptide precursor of the hormone calcitonin, the latter being involved with calcium homeostasis. It arises once preprocalcitonin is cleaved by endopeptidase. It was first identified by Leonard J. Deftos and Bernard A. Roos in the 1970s. It is composed of 116 amino acids and is produced by parafollicular cells of the thyroid and by the neuroendocrine cells of the lung and the intestine.
Stress hyperglycemia is a medical term referring to transient elevation of the blood glucose due to the stress of illness. It usually resolves spontaneously, but must be distinguished from various forms of diabetes mellitus.
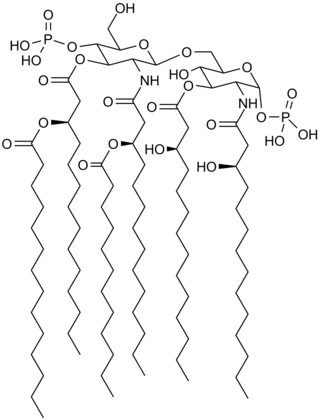
Lipid A is a lipid component of an endotoxin held responsible for the toxicity of gram-negative bacteria. It is the innermost of the three regions of the lipopolysaccharide (LPS), also called endotoxin molecule, and its hydrophobic nature allows it to anchor the LPS to the outer membrane. While its toxic effects can be damaging, the sensing of lipid A by the immune system may also be critical for the onset of immune responses to gram-negative infection, and for the subsequent successful fight against the infection.
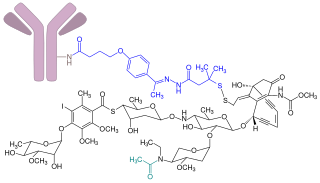
Gemtuzumab ozogamicin, sold under the brand name Mylotarg, is an antibody-drug conjugate that is used to treat acute myeloid leukemia.
The sequential organ failure assessment score, previously known as the sepsis-related organ failure assessment score, is used to track a person's status during the stay in an intensive care unit (ICU) to determine the extent of a person's organ function or rate of failure. The score is based on six different scores, one each for the respiratory, cardiovascular, hepatic, coagulation, renal and neurological systems.
A TNF inhibitor is a pharmaceutical drug that suppresses the physiologic response to tumor necrosis factor (TNF), which is part of the inflammatory response. TNF is involved in autoimmune and immune-mediated disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa and refractory asthma, so TNF inhibitors may be used in their treatment. The important side effects of TNF inhibitors include lymphomas, infections, congestive heart failure, demyelinating disease, a lupus-like syndrome, induction of auto-antibodies, injection site reactions, and systemic side effects.

Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack those cells. Alternatively, in radioimmunotherapy a radioactive dose localizes a target cell line, delivering lethal chemical doses. Antibodies have been used to bind to molecules involved in T-cell regulation to remove inhibitory pathways that block T-cell responses. This is known as immune checkpoint therapy.
Pagibaximab is a chimeric monoclonal antibody for the prevention of staphylococcal sepsis in infants with low birth weight. As of March 2010, it is undergoing Phase II/III clinical trials.
A clinical prediction rule or clinical probability assessment specifies how to use medical signs, symptoms, and other findings to estimate the probability of a specific disease or clinical outcome.
Critical illness–related corticosteroid insufficiency is a form of adrenal insufficiency in critically ill patients who have blood corticosteroid levels which are inadequate for the severe stress response they experience. Combined with decreased glucocorticoid receptor sensitivity and tissue response to corticosteroids, this adrenal insufficiency constitutes a negative prognostic factor for intensive care patients.
Nebacumab is a human monoclonal antibody developed for the treatment of sepsis. It has been withdrawn in 1993 because it failed to reduce mortality in clinical trials.
Vedolizumab, sold under the brand name Entyvio, is a monoclonal antibody medication developed by Millennium Pharmaceuticals, Inc. for the treatment of ulcerative colitis and Crohn's disease. It binds to integrin α4β7. Blocking the α4β7 integrin results in gut-selective anti-inflammatory activity.
Solanezumab is a monoclonal antibody being investigated by Eli Lilly as a neuroprotector for patients with Alzheimer's disease. The drug originally attracted extensive media coverage proclaiming it a breakthrough, but it has failed to show promise in Phase III trials.

Eritoran is an investigational drug for the treatment of severe sepsis, an excessive inflammatory response to an infection.
Didier Pittet is an infectious diseases expert and the director of the Infection Control Programme and WHO Collaborating Centre on Patient Safety, University Hospital of Geneva, Geneva, Switzerland. Since 2005, Pittet is also the External Lead of the World Health Organization (WHO) Global Patient Safety Challenge "Clean Care is Safer Care" and African Partnerships for Patient Safety.
Evolocumab is a monoclonal antibody medication designed for the treatment of hyperlipidemia.
Dostarlimab, sold under the brand name Jemperli, is a monoclonal antibody used as an anti-cancer medication for the treatment of endometrial cancer. Dostarlimab is a programmed death receptor-1 (PD-1)–blocking monoclonal antibody.

Convalescent plasma is the blood plasma collected from a survivor of an infectious disease. This plasma contains antibodies specific to a pathogen and can be used therapeutically by providing passive immunity when transfusing it to a newly infected patient with the same condition. Convalescent plasma can be transfused as it has been collected or become the source material for the hyperimmune serum which consists largely of IgG but also includes IgA and IgM. or as source material for anti-pathogen monoclonal antibodies, Collection is typically achieved by apheresis, but in low-to-middle income countries, the treatment can be administered as convalescent whole blood.







