Related Research Articles
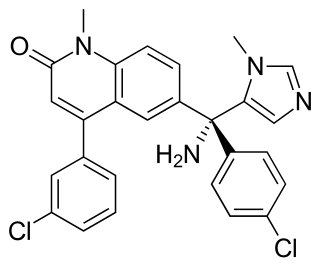
Tipifarnib is a farnesyltransferase inhibitor. Farnesyltransferase inhibitors block the activity of the farnesyltransferase enzyme by inhibiting prenylation of the CAAX tail motif, which ultimately prevents Ras from binding to the membrane, rendering it inactive.

Amrubicin is an anthracycline used in the treatment of lung cancer. It is marketed in Japan since 2002 by Sumitomo under the brand name Calsed.

Nafoxidine or nafoxidine hydrochloride (USAN) is a nonsteroidal selective estrogen receptor modulator (SERM) or partial antiestrogen of the triphenylethylene group that was developed for the treatment of advanced breast cancer by Upjohn in the 1970s but was never marketed. It was developed at around the same time as tamoxifen and clomifene, which are also triphenylethylene derivatives. The drug was originally synthesized by the fertility control program at Upjohn as a postcoital contraceptive, but was subsequently repurposed for the treatment of breast cancer. Nafoxidine was assessed in clinical trials in the treatment of breast cancer and was found to be effective. However, it produced side effects including ichthyosis, partial hair loss, and phototoxicity of the skin in almost all patients, and this resulted in the discontinuation of its development.
Lifastuzumab vedotin is an experimental monoclonal antibody-drug conjugate designed for the treatment of cancer.
Sofituzumab vedotin is a monoclonal antibody designed for the treatment of ovarian cancer.
Seribantumab is a monoclonal antibody designed for the treatment of cancer.
Brontictuzumab is a humanized monoclonal antibody designed for the treatment of cancer.
Lumretuzumab is a humanized monoclonal antibody designed for the treatment of cancer.
Vanucizumab is an experimental humanized monoclonal antibody designed for the treatment of cancer.
Vandortuzumab vedotin is a humanized monoclonal antibody designed for the treatment of cancer.
Depatuxizumab mafodotin is an antibody-drug conjugate designed for the treatment of cancer. It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.

Brilanestrant (INN) is a nonsteroidal combined selective estrogen receptor modulator (SERM) and selective estrogen receptor degrader (SERD) that was discovered by Aragon Pharmaceuticals and was under development by Genentech for the treatment of locally advanced or metastatic estrogen receptor (ER)-positive breast cancer.
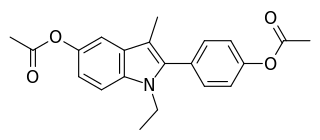
Zindoxifene is a nonsteroidal selective estrogen receptor modulator (SERM) that was under development in the 1980s and early 1990s for the treatment of breast cancer but was not marketed. It showed estrogenic-like activity in preclinical studies and failed to demonstrate effectiveness as a treatment for breast cancer in clinical trials. Zindoxifene was the lead compound of the distinct 2-phenylindole class of SERMs, and the marketed SERM bazedoxifene was derived from the major active metabolite of zindoxifene, D-15414. Zindoxifene was first described in 1984.
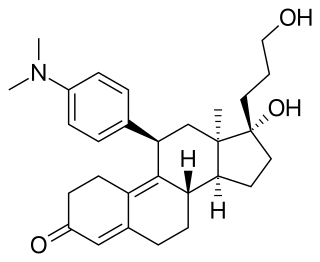
Onapristone (INN) is a synthetic and steroidal antiprogestogen with additional antiglucocorticoid activity which was developed by Schering and described in 1984 but was never marketed. It is a silent antagonist of the progesterone receptor (PR), in contrast to the related antiprogestogen mifepristone. Moreover, compared to mifepristone, onapristone has reduced antiglucocorticoid activity, shows little antiandrogenic activity, and has 10- to 30-fold greater potency as an antiprogestogen. The medication was under development for clinical use, for instance in the treatment of breast cancer and as an endometrial contraceptive, but was discontinued during phase III clinical trials in 1995 due to findings that liver function abnormalities developed in a majority patients.
Oleclumab is a human monoclonal antibody targeting the ectonucleotidase CD73 that was designed for the treatment of pancreatic and colorectal and other cancers.
Pamrevlumab is a humanized monoclonal antibody designed for the treatment of idiopathic pulmonary fibrosis and pancreatic cancer. It binds to the connective tissue growth factor (CTGF) protein.
Andecaliximab is an experimental humanized monoclonal antibody designed for the treatment of cancer and inflammatory diseases.
Rosmantuzumab is a humanized monoclonal antibody designed for the treatment of cancer.
Cofetuzumab pelidotin is an experimental antibody-drug conjugate in development for the treatment of cancer. It was created by Stemcentrx and is being developed by Pfizer. The drug is an anti-PTK7 monoclonal antibody linked to auristatin-0101, an auristatin microtubule inhibitor.

Rezvilutamide (INN), sold under the brand name Ariane, is a nonsteroidal antiandrogen which is approved for the treatment of prostate cancer in China and is or was under development for the treatment of breast cancer. It is a selective androgen receptor antagonist with reduced brain distribution compared to the structurally related nonsteroidal antiandrogen enzalutamide. The drug was developed by Jiangsu Hengrui Medicine. Other structural analogues of rezvilutamide that are also used as antiandrogens besides enzalutamide include apalutamide and proxalutamide.
References
- ↑ World Health Organization (2012). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 107" (PDF). WHO Drug Information. 26 (2).
- ↑ "Duligotuzumab". AdisInsight.