Adenosine triphosphate (ATP) is a nucleotide that provides energy to drive and support many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, it is often referred to as the "molecular unit of currency" of intracellular energy transfer.

Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is so pervasive because it releases more energy than alternative fermentation processes such as anaerobic glycolysis.
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. Many of the enzymes in the electron transport chain are embedded within the membrane.

Cellular respiration is the process by which biological fuels are oxidized in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.

ATPases (EC 3.6.1.3, Adenosine 5'-TriPhosphatase, adenylpyrophosphatase, ATP monophosphatase, triphosphatase, SV40 T-antigen, ATP hydrolase, complex V (mitochondrial electron transport), (Ca2+ + Mg2+)-ATPase, HCO3−-ATPase, adenosine triphosphatase) are a class of enzymes that catalyze the decomposition of ATP into ADP and a free phosphate ion or the inverse reaction. This dephosphorylation reaction releases energy, which the enzyme (in most cases) harnesses to drive other chemical reactions that would not otherwise occur. This process is widely used in all known forms of life.

ATP synthase is an enzyme that catalyzes the formation of the energy storage molecule adenosine triphosphate (ATP) using adenosine diphosphate (ADP) and inorganic phosphate (Pi). ATP synthase is a molecular machine. The overall reaction catalyzed by ATP synthase is:

Chemiosmosis is the movement of ions across a semipermeable membrane bound structure, down their electrochemical gradient. An important example is the formation of adenosine triphosphate (ATP) by the movement of hydrogen ions (H+) across a membrane during cellular respiration or photosynthesis.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.

F-ATPase, also known as F-Type ATPase, is an ATPase/synthase found in bacterial plasma membranes, in mitochondrial inner membranes, and in chloroplast thylakoid membranes. It uses a proton gradient to drive ATP synthesis by allowing the passive flux of protons across the membrane down their electrochemical gradient and using the energy released by the transport reaction to release newly formed ATP from the active site of F-ATPase. Together with V-ATPases and A-ATPases, F-ATPases belong to superfamily of related rotary ATPases.

Antimycin A is a secondary metabolite produced by Streptomyces bacteria and a member of a group of related compounds called antimycins. Antimycin A is classified as an extremely hazardous substance in the United States, as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.

An uncoupling protein (UCP) is a mitochondrial inner membrane protein that is a regulated proton channel or transporter. An uncoupling protein is thus capable of dissipating the proton gradient generated by NADH-powered pumping of protons from the mitochondrial matrix to the mitochondrial intermembrane space. The energy lost in dissipating the proton gradient via UCPs is not used to do biochemical work. Instead, heat is generated. This is what links UCP to thermogenesis. However, not every type of UCPs are related to thermogenesis. Although UCP2 and UCP3 are closely related to UCP1, UCP2 and UCP3 do not affect thermoregulatory abilities of vertebrates. UCPs are positioned in the same membrane as the ATP synthase, which is also a proton channel. The two proteins thus work in parallel with one generating heat and the other generating ATP from ADP and inorganic phosphate, the last step in oxidative phosphorylation. Mitochondria respiration is coupled to ATP synthesis, but is regulated by UCPs. UCPs belong to the mitochondrial carrier (SLC25) family.

MT-ATP8 is a mitochondrial gene with the full name 'mitochondrially encoded ATP synthase membrane subunit 8' that encodes a subunit of mitochondrial ATP synthase, ATP synthase Fo subunit 8. This subunit belongs to the Fo complex of the large, transmembrane F-type ATP synthase. This enzyme, which is also known as complex V, is responsible for the final step of oxidative phosphorylation in the electron transport chain. Specifically, one segment of ATP synthase allows positively charged ions, called protons, to flow across a specialized membrane inside mitochondria. Another segment of the enzyme uses the energy created by this proton flow to convert a molecule called adenosine diphosphate (ADP) to ATP. Subunit 8 differs in sequence between Metazoa, plants and Fungi.
MT-ATP6 is a mitochondrial gene with the full name 'mitochondrially encoded ATP synthase membrane subunit 6' that encodes the ATP synthase Fo subunit 6. This subunit belongs to the Fo complex of the large, transmembrane F-type ATP synthase. This enzyme, which is also known as complex V, is responsible for the final step of oxidative phosphorylation in the electron transport chain. Specifically, one segment of ATP synthase allows positively charged ions, called protons, to flow across a specialized membrane inside mitochondria. Another segment of the enzyme uses the energy created by this proton flow to convert a molecule called adenosine diphosphate (ADP) to ATP. Mutations in the MT-ATP6 gene have been found in approximately 10 to 20 percent of people with Leigh syndrome.

ATP synthase F1 subunit alpha, mitochondrial is an enzyme that in humans is encoded by the ATP5F1A gene.

Brain mitochondrial carrier protein 1 is a protein that in humans is encoded by the SLC25A14 gene.

ATP synthase subunit delta, mitochondrial, also known as ATP synthase F1 subunit delta or F-ATPase delta subunit is an enzyme that in humans is encoded by the ATP5F1D gene. This gene encodes a subunit of mitochondrial ATP synthase. Mitochondrial ATP synthase catalyzes ATP synthesis, utilizing an electrochemical gradient of protons across the inner membrane during oxidative phosphorylation.

Dinoseb is a common industry name for 6-sec-butyl-2,4-dinitrophenol, a herbicide in the dinitrophenol family. It is a crystalline orange solid which does not readily dissolve in water. Dinoseb is banned as an herbicide in the European Union (EU) and the United States because of its toxicity.
An uncoupler or uncoupling agent is a molecule that disrupts oxidative phosphorylation in prokaryotes and mitochondria or photophosphorylation in chloroplasts and cyanobacteria by dissociating the reactions of ATP synthesis from the electron transport chain. The result is that the cell or mitochondrion expends energy to generate a proton-motive force, but the proton-motive force is dissipated before the ATP synthase can recapture this energy and use it to make ATP. Because the intracellular supply of protons is replenished, uncouplers actually stimulate cellular metabolism. Uncouplers are capable of transporting protons through mitochondrial and lipid membranes.
Fish acute toxicity syndrome (FATS) is a set of common chemical and functional responses in fish resulting from a short-term, acute exposure to a lethal concentration of a toxicant, a chemical or material that can produce an unfavorable effect in a living organism. By definition, modes of action are characterized by FATS because the combination of common responses that represent each fish acute toxicity syndrome characterize an adverse biological effect. Therefore, toxicants that have the same mode of action elicit similar sets of responses in the organism and can be classified by the same fish acute toxicity syndrome.

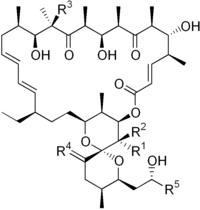
Dibutylchloromethyltin chloride (DBCT) is a toxic organotin compound. It's a potent and irreversible ATP synthase inhibitor. DBCT is a volatile liquid with powerful vesicant effects.