The mesoderm is the middle layer of the three germ layers that develops during gastrulation in the very early development of the embryo of most animals. The outer layer is the ectoderm, and the inner layer is the endoderm.
Transdifferentiation, also known as lineage reprogramming, is the process in which one mature somatic cell is transformed into another mature somatic cell without undergoing an intermediate pluripotent state or progenitor cell type. It is a type of metaplasia, which includes all cell fate switches, including the interconversion of stem cells. Current uses of transdifferentiation include disease modeling and drug discovery and in the future may include gene therapy and regenerative medicine. The term 'transdifferentiation' was originally coined by Selman and Kafatos in 1974 to describe a change in cell properties as cuticle producing cells became salt-secreting cells in silk moths undergoing metamorphosis.
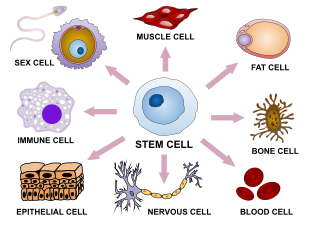
Cellular differentiation is the process in which a stem cell changes from one type to a differentiated one. Usually, the cell changes to a more specialized type. Differentiation happens multiple times during the development of a multicellular organism as it changes from a simple zygote to a complex system of tissues and cell types. Differentiation continues in adulthood as adult stem cells divide and create fully differentiated daughter cells during tissue repair and during normal cell turnover. Some differentiation occurs in response to antigen exposure. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. These changes are largely due to highly controlled modifications in gene expression and are the study of epigenetics. With a few exceptions, cellular differentiation almost never involves a change in the DNA sequence itself. However, metabolic composition does get altered quite dramatically where stem cells are characterized by abundant metabolites with highly unsaturated structures whose levels decrease upon differentiation. Thus, different cells can have very different physical characteristics despite having the same genome.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.
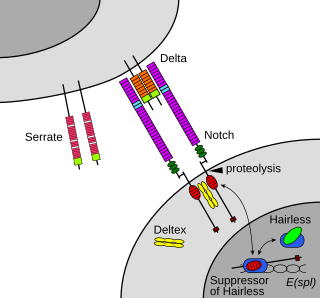
The Notch signaling pathway is a highly conserved cell signaling system present in most animals. Mammals possess four different notch receptors, referred to as NOTCH1, NOTCH2, NOTCH3, and NOTCH4. The notch receptor is a single-pass transmembrane receptor protein. It is a hetero-oligomer composed of a large extracellular portion, which associates in a calcium-dependent, non-covalent interaction with a smaller piece of the notch protein composed of a short extracellular region, a single transmembrane-pass, and a small intracellular region.

MyoD, also known as myoblast determination protein 1, is a protein in animals that plays a major role in regulating muscle differentiation. MyoD, which was discovered in the laboratory of Harold M. Weintraub, belongs to a family of proteins known as myogenic regulatory factors (MRFs). These bHLH transcription factors act sequentially in myogenic differentiation. Vertebrate MRF family members include MyoD1, Myf5, myogenin, and MRF4 (Myf6). In non-vertebrate animals, a single MyoD protein is typically found.

Myogenesis is the formation of skeletal muscular tissue, particularly during embryonic development.

mir-133 is a type of non-coding RNA called a microRNA that was first experimentally characterised in mice. Homologues have since been discovered in several other species including invertebrates such as the fruitfly Drosophila melanogaster. Each species often encodes multiple microRNAs with identical or similar mature sequence. For example, in the human genome there are three known miR-133 genes: miR-133a-1, miR-133a-2 and miR-133b found on chromosomes 18, 20 and 6 respectively. The mature sequence is excised from the 3' arm of the hairpin. miR-133 is expressed in muscle tissue and appears to repress the expression of non-muscle genes.

Retinoic acid receptor alpha (RAR-α), also known as NR1B1, is a nuclear receptor that in humans is encoded by the RARA gene.

Retinoic acid receptor beta (RAR-beta), also known as NR1B2 is a nuclear receptor that in humans is encoded by the RARB gene.

Retinoic acid-induced protein 3 is a protein that in humans is encoded by the GPRC5A gene. This gene and its encoded mRNA was first identified as a phorbol ester-induced gene, and named Phorbol Ester Induced Gen 1 (PEIG-1); two years later it was rediscovered as a retinoic acid-inducible gene, and named Retinoic Acid-Inducible Gene 1 (RAIG1). Its encoded protein was later named Retinoic acid-induced protein 3.

Cellular retinoic acid-binding protein 2 is a cytoplasmic binding protein that in humans is encoded by the CRABP2 gene.

Cytochrome P450 26A1 is a protein that in humans is encoded by the CYP26A1 gene.

Cellular retinoic acid-binding protein 1 is a protein that in humans is encoded by the CRABP1 gene.

Aldehyde dehydrogenase 1 family, member A2, also known as ALDH1A2 or retinaldehyde dehydrogenase 2 (RALDH2), is an enzyme that in humans is encoded by the ALDH1A2 gene.
Lin-28 homolog A is a protein that in humans is encoded by the LIN28 gene.

Rex1 (Zfp-42) is a known marker of pluripotency, and is usually found in undifferentiated embryonic stem cells. In addition to being a marker for pluripotency, its regulation is also critical in maintaining a pluripotent state. As the cells begin to differentiate, Rex1 is severely and abruptly downregulated.
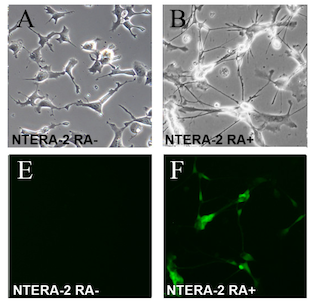
The NTERA-2 cell line is a clonally derived, pluripotent human embryonal carcinoma cell line.
Directed differentiation is a bioengineering methodology at the interface of stem cell biology, developmental biology and tissue engineering. It is essentially harnessing the potential of stem cells by constraining their differentiation in vitro toward a specific cell type or tissue of interest. Stem cells are by definition pluripotent, able to differentiate into several cell types such as neurons, cardiomyocytes, hepatocytes, etc. Efficient directed differentiation requires a detailed understanding of the lineage and cell fate decision, often provided by developmental biology.
Cardiomyocyte proliferation refers to the ability of cardiac muscle cells to progress through the cell cycle and continue to divide. Traditionally, cardiomyocytes were believed to have little to no ability to proliferate and regenerate after birth. Although other types of cells, such as gastrointestinal epithelial cells, can proliferate and differentiate throughout life, cardiac tissue contains little intrinsic ability to proliferate, as adult human cells arrest in the cell cycle. However, a recent paradigm shift has occurred. Recent research has demonstrated that human cardiomyocytes do proliferate to a small extent for the first two decades of life. Also, cardiomyocyte proliferation and regeneration has been demonstrated to occur in various neonatal mammals in response to injury in the first week of life. Current research aims to further understand the biological mechanism underlying cardiomyocyte proliferation in hopes to turn this capability back on in adults in order to combat heart disease.