
The citric acid cycle—also known as the Krebs cycle, Szent-Györgyi-Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of biochemical reactions to release the energy stored in nutrients through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The chemical energy released is available under the form of ATP. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.

Glycolysis is the metabolic pathway that converts glucose into pyruvate and, in most organisms, occurs in the liquid part of cells. The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes.

In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell.
Gluconeogenesis (GNG) is a metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen (glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise.

Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. Pyruvate kinase was inappropriately named before it was recognized that it did not directly catalyze phosphorylation of pyruvate, which does not occur under physiological conditions. Pyruvate kinase is present in four distinct, tissue-specific isozymes in animals, each consisting of particular kinetic properties necessary to accommodate the variations in metabolic requirements of diverse tissues.

Mixed inhibition is a type of enzyme inhibition in which the inhibitor may bind to the enzyme whether or not the enzyme has already bound the substrate but has a greater affinity for one state or the other. It is called "mixed" because it can be seen as a conceptual "mixture" of competitive inhibition, in which the inhibitor can only bind the enzyme if the substrate has not already bound, and uncompetitive inhibition, in which the inhibitor can only bind the enzyme if the substrate has already bound. If the ability of the inhibitor to bind the enzyme is exactly the same whether or not the enzyme has already bound the substrate, it is known as a non-competitive inhibitor. Non-competitive inhibition is sometimes thought of as a special case of mixed inhibition.
Substrate-level phosphorylation is a metabolism reaction that results in the production of ATP or GTP supported by the energy released from another high-energy bond that leads to phosphorylation of ADP or GDP to ATP or GTP (note that the reaction catalyzed by creatine kinase is not considered as "substrate-level phosphorylation"). This process uses some of the released chemical energy, the Gibbs free energy, to transfer a phosphoryl (PO3) group to ADP or GDP. Occurs in glycolysis and in the citric acid cycle.

Pyruvate carboxylase (PC) encoded by the gene PC is an enzyme of the ligase class that catalyzes the physiologically irreversible carboxylation of pyruvate to form oxaloacetate (OAA).

Phosphoenolpyruvate is the carboxylic acid derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found in organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system.
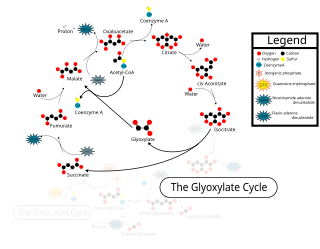
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.

Phosphoenolpyruvate carboxykinase is an enzyme in the lyase family used in the metabolic pathway of gluconeogenesis. It converts oxaloacetate into phosphoenolpyruvate and carbon dioxide.
Phosphoenolpyruvate carboxykinase 1 (soluble), also known as PCK1, is an enzyme which in humans is encoded by the PCK1 gene.

28S ribosomal protein S24, mitochondrial is a protein that in humans is encoded by the MRPS24 gene.

Enolase 3 (ENO3), more commonly known as beta-enolase (ENO-β), is an enzyme that in humans is encoded by the ENO3 gene.

Pyruvate kinase isozymes M1/M2 (PKM1/M2), also known as pyruvate kinase muscle isozyme (PKM), pyruvate kinase type K, cytosolic thyroid hormone-binding protein (CTHBP), thyroid hormone-binding protein 1 (THBP1), or opa-interacting protein 3 (OIP3), is an enzyme that in humans is encoded by the PKM2 gene.
Pyruvate cycling commonly refers to an intracellular loop of spatial movements and chemical transformations involving pyruvate. Spatial movements occur between mitochondria and cytosol and chemical transformations create various Krebs cycle intermediates. In all variants, pyruvate is imported into the mitochondrion for processing through part of the Krebs cycle. In addition to pyruvate, alpha-ketoglutarate may also be imported. At various points, the intermediate product is exported to the cytosol for additional transformations and then re-imported. Three specific pyruvate cycles are generally considered, each named for the principal molecule exported from the mitochondrion: malate, citrate, and isocitrate. Other variants may exist, such as dissipative or "futile" pyruvate cycles.
Glyceroneogenesis is a metabolic pathway which synthesizes glycerol 3-phosphate from precursors other than glucose. Usually, glycerol 3-phosphate is generated from glucose by glycolysis, in the liquid of the cell's cytoplasm. Glyceroneogenesis is used when the concentrations of glucose in the cytosol are low, and typically uses pyruvate as the precursor, but can also use alanine, glutamine, or any substances from the TCA cycle. The main regulator enzyme for this pathway is an enzyme called phosphoenolpyruvate carboxykinase (PEPC-K), which catalyzes the decarboxylation of oxaloacetate to phosphoenolpyruvate. Glyceroneogenesis is observed mainly in adipose tissue, and in the liver. A significant biochemical pathway regulates cytosolic lipid levels. Intense suppression of glyceroneogenesis may lead to metabolic disorders such as type 2 diabetes.

Mitochondrial ribosomal protein L3 is a protein that in humans is encoded by the MRPL3 gene.

Phosphoglycerate mutase 2 (PGAM2), also known as muscle-specific phosphoglycerate mutase (PGAM-M), is a phosphoglycerate mutase that, in humans, is encoded by the PGAM2 gene on chromosome 7.

Succinyl-CoA ligase [GDP-forming] subunit beta, mitochondrial is an enzyme that in humans is encoded by the SUCLG2 gene on chromosome 3.


















