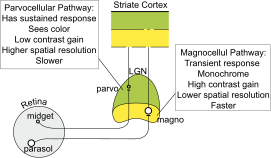
The visual cortex of the brain is the area of the cerebral cortex that processes visual information. It is located in the occipital lobe. Sensory input originating from the eyes travels through the lateral geniculate nucleus in the thalamus and then reaches the visual cortex. The area of the visual cortex that receives the sensory input from the lateral geniculate nucleus is the primary visual cortex, also known as visual area 1 (V1), Brodmann area 17, or the striate cortex. The extrastriate areas consist of visual areas 2, 3, 4, and 5.

The retina is the innermost, light-sensitive layer of tissue of the eye of most vertebrates and some molluscs. The optics of the eye create a focused two-dimensional image of the visual world on the retina, which then processes that image within the retina and sends nerve impulses along the optic nerve to the visual cortex to create visual perception. The retina serves a function which is in many ways analogous to that of the film or image sensor in a camera.

The visual system is the physiological basis of visual perception. The system detects, transduces and interprets information concerning light within the visible range to construct an image and build a mental model of the surrounding environment. The visual system is associated with the eye and functionally divided into the optical system and the neural system.

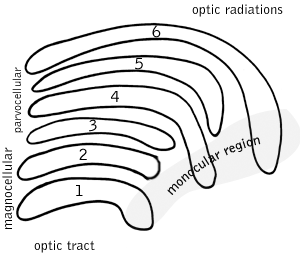
In neuroanatomy, the lateral geniculate nucleus is a structure in the thalamus and a key component of the mammalian visual pathway. It is a small, ovoid, ventral projection of the thalamus where the thalamus connects with the optic nerve. There are two LGNs, one on the left and another on the right side of the thalamus. In humans, both LGNs have six layers of neurons alternating with optic fibers.

A retinal ganglion cell (RGC) is a type of neuron located near the inner surface of the retina of the eye. It receives visual information from photoreceptors via two intermediate neuron types: bipolar cells and retina amacrine cells. Retina amacrine cells, particularly narrow field cells, are important for creating functional subunits within the ganglion cell layer and making it so that ganglion cells can observe a small dot moving a small distance. Retinal ganglion cells collectively transmit image-forming and non-image forming visual information from the retina in the form of action potential to several regions in the thalamus, hypothalamus, and mesencephalon, or midbrain.
The receptive field, or sensory space, is a delimited medium where some physiological stimuli can evoke a sensory neuronal response in specific organisms.
In neuroscience, parvocellular cells, also called P-cells, are neurons located within the parvocellular layers of the lateral geniculate nucleus (LGN) of the thalamus. Their name comes from Latin parvus 'small', due to the small size of the cell compared to the larger magnocellular cells. Phylogenetically, parvocellular neurons are more modern than magnocellular ones.
Magnocellular cells, also called M-cells, are neurons located within the magnocellular layer of the lateral geniculate nucleus of the thalamus. The cells are part of the visual system. They are termed "magnocellular" since they are characterized by their relatively large size compared to parvocellular cells.
The opponent process is a color theory that states that the human visual system interprets information about color by processing signals from photoreceptor cells in an antagonistic manner. The opponent-process theory suggests that there are three opponent channels, each comprising an opposing color pair: red versus green, blue versus yellow, and black versus white (luminance). The theory was first proposed in 1892 by the German physiologist Ewald Hering.

Motion perception is the process of inferring the speed and direction of elements in a scene based on visual, vestibular and proprioceptive inputs. Although this process appears straightforward to most observers, it has proven to be a difficult problem from a computational perspective, and difficult to explain in terms of neural processing.
In neuroscience, a koniocellular cell is a neuron with a small cell body that is located in the koniocellular layer of the lateral geniculate nucleus (LGN) of the thalamus of primates, including humans.
Intrinsically photosensitive retinal ganglion cells (ipRGCs), also called photosensitive retinal ganglion cells (pRGC), or melanopsin-containing retinal ganglion cells (mRGCs), are a type of neuron in the retina of the mammalian eye. The presence of an additional photoreceptor was first suspected in 1927 when mice lacking rods and cones still responded to changing light levels through pupil constriction; this suggested that rods and cones are not the only light-sensitive tissue. However, it was unclear whether this light sensitivity arose from an additional retinal photoreceptor or elsewhere in the body. Recent research has shown that these retinal ganglion cells, unlike other retinal ganglion cells, are intrinsically photosensitive due to the presence of melanopsin, a light-sensitive protein. Therefore, they constitute a third class of photoreceptors, in addition to rod and cone cells.

Retinotopy is the mapping of visual input from the retina to neurons, particularly those neurons within the visual stream. For clarity, 'retinotopy' can be replaced with 'retinal mapping', and 'retinotopic' with 'retinally mapped'.
Blobs are sections of primary visual cortex above and below layer IV where groups of neurons sensitive to color assemble in cylindrical shapes. They were first identified in 1979 by Margaret Wong-Riley when she used a cytochrome oxidase stain, from which they get their name. These areas receive input from koniocellular cells in the dorsal lateral geniculate nucleus dLGN and output to the thin stripes of area V2. Interblobs are areas between blobs that receive the same input, but are sensitive to orientation instead of color. They output to the pale and thick stripes of area V2.
Feature detection is a process by which the nervous system sorts or filters complex natural stimuli in order to extract behaviorally relevant cues that have a high probability of being associated with important objects or organisms in their environment, as opposed to irrelevant background or noise.

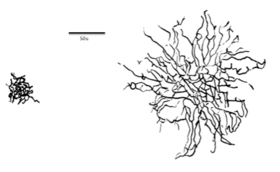
A midget cell, sometimes called a P cell or P ganglion cell, is one type of retinal ganglion cell (RGC). Midget cells originate in the ganglion cell layer of the retina, and project to the parvocellular layers of the lateral geniculate nucleus (LGN). The axons of midget cells travel through the optic nerve and optic tract, ultimately synapsing with parvocellular cells in the LGN. These cells are known as midget retinal ganglion cells due to the small sizes of their dendritic trees and cell bodies. About 80% of RGCs are midget cells. They receive inputs from relatively few rods and cones. In many cases, they are connected to midget bipolar cells, which are linked to one cone each.

A phantom contour is a type of illusory contour. Most illusory contours are seen in still images, such as the Kanizsa triangle and the Ehrenstein illusion. A phantom contour, however, is perceived in the presence of moving or flickering images with contrast reversal. The rapid, continuous alternation between opposing, but correlated, adjacent images creates the perception of a contour that is not physically present in the still images. Quaid et al. have also authored a PhD thesis on the phantom contour illusion and its spatiotemporal limits which maps out limits and proposes mechanisms for its perception centering around magnocellularly driven visual area MT.
Retinal waves are spontaneous bursts of action potentials that propagate in a wave-like fashion across the developing retina. These waves occur before rod and cone maturation and before vision can occur. The signals from retinal waves drive the activity in the dorsal lateral geniculate nucleus (dLGN) and the primary visual cortex. The waves are thought to propagate across neighboring cells in random directions determined by periods of refractoriness that follow the initial depolarization. Retinal waves are thought to have properties that define early connectivity of circuits and synapses between cells in the retina. There is still much debate about the exact role of retinal waves. Some contend that the waves are instructional in the formation of retinogeniculate pathways, while others argue that the activity is necessary but not instructional in the formation of retinogeniculate pathways.

Peter H. Schiller was a German-born neuroscientist. At the time of his death, he was a professor emeritus of Neuroscience in the Department of Brain and Cognitive Sciences at the Massachusetts Institute of Technology (MIT). Schiller is well known for his work on the behavioral, neurophysiological and pharmacological studies of the primate visual and oculomotor systems.

Laura Busse is a German neuroscientist and professor of Systemic Neuroscience within the Division of Neurobiology at the Ludwig Maximilian University of Munich. Busse's lab studies context-dependent visual processing in mouse models by performing large scale in vivo electrophysiological recordings in the thalamic and cortical circuits of awake and behaving mice.