Related Research Articles

The stomach is a muscular, hollow organ in the gastrointestinal tract of humans and many other animals, including several invertebrates. The stomach has a dilated structure and functions as a vital organ in the digestive system. The stomach is involved in the gastric phase of digestion, following chewing. It performs a chemical breakdown by means of enzymes and hydrochloric acid.

Chymosin or rennin is a protease found in rennet. It is an aspartic endopeptidase belonging to MEROPS A1 family. It is produced by newborn ruminant animals in the lining of the abomasum to curdle the milk they ingest, allowing a longer residence in the bowels and better absorption. It is widely used in the production of cheese.
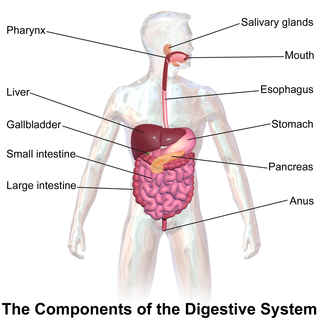
Digestion is the breakdown of large insoluble food molecules into small water-soluble food molecules so that they can be absorbed into the watery blood plasma. In certain organisms, these smaller substances are absorbed through the small intestine into the blood stream. Digestion is a form of catabolism that is often divided into two processes based on how food is broken down: mechanical and chemical digestion. The term mechanical digestion refers to the physical breakdown of large pieces of food into smaller pieces which can subsequently be accessed by digestive enzymes. Mechanical digestion takes place in the mouth through mastication and in the small intestine through segmentation contractions. In chemical digestion, enzymes break down food into the small molecules the body can use.
Pepsin is an endopeptidase that breaks down proteins into smaller peptides. It is produced in the gastric chief cells of the stomach lining and is one of the main digestive enzymes in the digestive systems of humans and many other animals, where it helps digest the proteins in food. Pepsin is an aspartic protease, using a catalytic aspartate in its active site.
In biochemistry, a zymogen, also called a proenzyme, is an inactive precursor of an enzyme. A zymogen requires a biochemical change for it to become an active enzyme. The biochemical change usually occurs in Golgi bodies, where a specific part of the precursor enzyme is cleaved in order to activate it. The inactivating piece which is cleaved off can be a peptide unit, or can be independently-folding domains comprising more than 100 residues. Although they limit the enzyme's ability, these N-terminal extensions of the enzyme or a “prosegment” often aid in the stabilization and folding of the enzyme they inhibit.

An exoenzyme, or extracellular enzyme, is an enzyme that is secreted by a cell and functions outside that cell. Exoenzymes are produced by both prokaryotic and eukaryotic cells and have been shown to be a crucial component of many biological processes. Most often these enzymes are involved in the breakdown of larger macromolecules. The breakdown of these larger macromolecules is critical for allowing their constituents to pass through the cell membrane and enter into the cell. For humans and other complex organisms, this process is best characterized by the digestive system which breaks down solid food via exoenzymes. The small molecules, generated by the exoenzyme activity, enter into cells and are utilized for various cellular functions. Bacteria and fungi also produce exoenzymes to digest nutrients in their environment, and these organisms can be used to conduct laboratory assays to identify the presence and function of such exoenzymes. Some pathogenic species also use exoenzymes as virulence factors to assist in the spread of these disease-causing microorganisms. In addition to the integral roles in biological systems, different classes of microbial exoenzymes have been used by humans since pre-historic times for such diverse purposes as food production, biofuels, textile production and in the paper industry. Another important role that microbial exoenzymes serve is in the natural ecology and bioremediation of terrestrial and marine environments.

John Howard Northrop was an American biochemist who, with James Batcheller Sumner and Wendell Meredith Stanley, won the 1946 Nobel Prize in Chemistry. The award was given for these scientists' isolation, crystallization, and study of enzymes, proteins, and viruses. Northrop was a Professor of Bacteriology and Medical Physics, Emeritus, at University of California, Berkeley.

Gastric acid, gastric juice, or stomach acid is a digestive fluid formed within the stomach lining. With a pH between 1 and 3, gastric acid plays a key role in digestion of proteins by activating digestive enzymes, which together break down the long chains of amino acids of proteins. Gastric acid is regulated in feedback systems to increase production when needed, such as after a meal. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid, ensuring a regulated pH. These cells also produce mucus – a viscous barrier to prevent gastric acid from damaging the stomach. The pancreas further produces large amounts of bicarbonate and secretes bicarbonate through the pancreatic duct to the duodenum to neutralize gastric acid passing into the digestive tract.

Digestive enzymes are a group of enzymes that break down polymeric macromolecules into their smaller building blocks, in order to facilitate their absorption into the cells of the body. Digestive enzymes are found in the digestive tracts of animals and in the tracts of carnivorous plants, where they aid in the digestion of food, as well as inside cells, especially in their lysosomes, where they function to maintain cellular survival. Digestive enzymes of diverse specificities are found in the saliva secreted by the salivary glands, in the secretions of cells lining the stomach, in the pancreatic juice secreted by pancreatic exocrine cells, and in the secretions of cells lining the small and large intestines.
A proteose is any of various water-soluble compounds that are produced during in-vitro or in-vivo hydrolytic breakdown of proteins a little before producing amino acids. It forms after breaking down of polypeptides by proteases such as gastric pepsin. In addition to proteoses, peptones are also formed at this stage. The difference between peptones and proteoses is that proteoses are precipitated from solution by half saturation with ammonium sulfate, while peptones don't react even with fully saturated ammonium sulfate.
In human anatomy, there are three types of chief cells, the gastric chief cell, the parathyroid chief cell, and the type 1 chief cells found in the carotid body.

Pepsin A is an enzyme. This enzyme catalyses the following chemical reaction

Aspartic proteases are a catalytic type of protease enzymes that use an activated water molecule bound to one or more aspartate residues for catalysis of their peptide substrates. In general, they have two highly conserved aspartates in the active site and are optimally active at acidic pH. Nearly all known aspartyl proteases are inhibited by pepstatin.
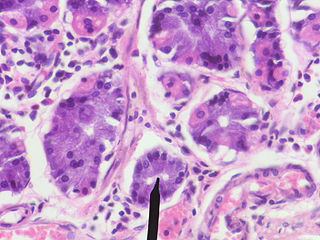
A gastric chief cell is a type of gastric gland cell that releases pepsinogen and gastric lipase. It is the cell responsible for secretion of chymosin in ruminant animals. The cell stains basophilic upon H&E staining due to the large proportion of rough endoplasmic reticulum in its cytoplasm. Gastric chief cells are generally located deep in the mucosal layer of the stomach lining, in the fundus and body of the stomach.
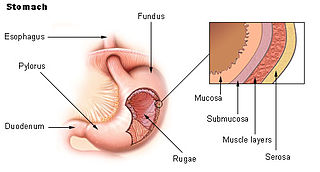
The gastric mucosa is the mucous membrane layer of the stomach, which contains the glands and the gastric pits. In humans, it is about 1 mm thick, and its surface is smooth, soft, and velvety. It consists of simple columnar epithelium, lamina propria, and the muscularis mucosae.
Gastrointestinal physiology is the branch of human physiology that addresses the physical function of the gastrointestinal (GI) tract. The function of the GI tract is to process ingested food by mechanical and chemical means, extract nutrients and excrete waste products. The GI tract is composed of the alimentary canal, that runs from the mouth to the anus, as well as the associated glands, chemicals, hormones, and enzymes that assist in digestion. The major processes that occur in the GI tract are: motility, secretion, regulation, digestion and circulation. The proper function and coordination of these processes are vital for maintaining good health by providing for the effective digestion and uptake of nutrients.
Progastricsin also known as pepsinogen C or pepsinogen II is a pepsinogen precursor of the enzyme gastricsin that in humans is encoded by the PGC gene.

Pepsinogen A is a protein that in humans is encoded by the PGA5 gene.

Gertrude Erika Perlmann was an Austro-Hungarian Empire-born U.S. biochemist and structural biologist. She is known for her work in protein chemistry, particularly her discoveries on the biology phosphoproteins and the structure and action of pepsin and pepsinogen.

Pepsinogen 3, group I is a protein that in humans is encoded by the PGA3 gene.
References
- ↑ Ryle AP (1970). Perlmann GE, Lorand, L (eds.). The porcine pepsins and pepsinogens. Methods in Enzymology. Vol. 19. pp. 316–336. doi:10.1016/0076-6879(70)19023-9.