The Swern oxidation, named after Daniel Swern, is a chemical reaction whereby a primary or secondary alcohol is oxidized to an aldehyde or ketone using oxalyl chloride, dimethyl sulfoxide (DMSO) and an organic base, such as triethylamine. It is one of the many oxidation reactions commonly referred to as 'activated DMSO' oxidations. The reaction is known for its mild character and wide tolerance of functional groups.

In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Perilla frutescensvar.crispa, also known by its Japanese name shiso, is a cultigen of Perilla frutescens, a herb in the mint family Lamiaceae. It is native to the mountainous regions of China and India, but is now found worldwide. The plant occurs in several forms, as defined by the characteristics of their leaves, including red, green, bicolor, and ruffled. Shiso is perennial and may be cultivated as an annual in temperate climates. Different parts of the plant are used in East Asian and Southeast Asian cuisine.
The Stille reaction is a chemical reaction widely used in organic synthesis. The reaction involves the coupling of two organic groups, one of which is carried as an organotin compound. A variety of organic electrophiles provide the other coupling partner. The Stille reaction is one of many palladium-catalyzed coupling reactions.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:
The Blaise ketone synthesis is the chemical reaction of acid chlorides with organozinc compounds to give ketones.

Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers.

Organozinc compounds in organic chemistry contain carbon (C) to zinc (Zn) chemical bonds. Organozinc chemistry is the science of organozinc compounds describing their physical properties, synthesis and reactions.

An organocadmium compound is an organometallic compound containing a carbon to cadmium chemical bond. Organocadmium chemistry describes physical properties, synthesis, reactions and use of these compounds. Cadmium shares group 12 with zinc and mercury and their corresponding chemistries have much in common. The synthetic utility of organocadmium compounds is limited.

Diphenyl diselenide is the chemical compound with the formula (C6H5)2Se2, abbreviated Ph2Se2. This orange-coloured solid is the oxidized derivative of benzeneselenol. It is used as a source of the PhSe unit in organic synthesis.

Perilla frutescens, commonly called deulkkae, perilla or Korean perilla, is a species of Perilla in the mint family Lamiaceae. It is an annual plant native to Southeast Asia and Indian highlands, and is traditionally grown in the Korean peninsula, southern China, Japan and India as a crop.
Selenoxide elimination is a method for the chemical synthesis of alkenes from selenoxides. It is most commonly used to synthesize α,β-unsaturated carbonyl compounds from the corresponding saturated analogues. It is mechanistically related to the Cope reaction.

Takai olefination in organic chemistry describes the organic reaction of an aldehyde with a diorganochromium compound to form an alkene. It is a name reaction, referencing Kazuhiko Takai, who first reported it in 1986. In the original reaction, the organochromium species is generated from iodoform or bromoform and an excess of chromium(II) chloride and the product is a vinyl halide. One main advantage of this reaction is the E-configuration of the double bond that is formed. According to the original report, existing alternatives such as the Wittig reaction only gave mixtures.

Tetramethyltin is an organometallic compound with the formula (CH3)4Sn. This liquid, one of the simplest organotin compounds, is useful for transition-metal mediated conversion of acid chlorides to methyl ketones and aryl halides to aryl methyl ketones. It is volatile and toxic, so care should be taken when using it in the laboratory.
The Abramov reaction is the related conversions of trialkyl to α-hydroxy phosphonates by the addition to carbonyl compounds. In terms of mechanism, the reaction involves attack of the nucleophilic phosphorus atom on the carbonyl carbon. It was named after the Russian chemist Vasilii Semenovich Abramov (1904–1968) in 1957.
Perilla may refer to any of the following subjects:
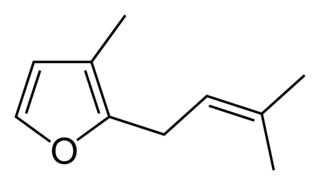
Rosefuran (3-methyl-2-prenylfuran) is a liquid boiling at 103-104 °C, with a density of 0.9089 g/cm3, less than that of water. It is an aroma chemical which is a minor constituent of the aroma of the rose. Rosefuran is a 2,3-disubstituted furan. It has an odor threshold of 200 ppb and constitutes 0.16% of Bulgarian rose oil. Rosefuran has been established as a female sex pheromone of an acarid mite, Caloglyphus sp. Concentrations of less than 100 ng of synthetic rosefuran caused sexual excitation in males of the species.
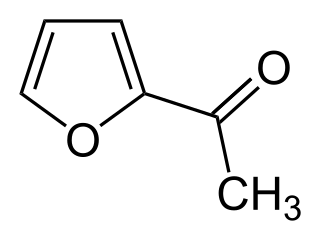
2-Acetylfuran is a low melting solid or high boiling liquid, depending on temperature. The solid melts at 30 °C and has a density of 1.0975 g/ml at 20 °C, while the normal boiling point of the liquid is 168–169 °C. 2-Acetylfuran is a useful intermediate in the synthesis of fine chemicals and pharmaceuticals, and is used in the production of the generic cephalophosphorin antibiotic cefuroxime.
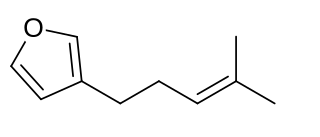
Perillene is a natural monoterpene that consists of a furan ring with a six-carbon homoprenyl side chain. Perillene is a component of the essential oil obtained by extraction of the leaves of Perilla frutescens. Perillene has also been obtained by steam distillation of the leaves of Perilla frutescens. Perillene has been found to elicit distinct electrophysiological responses in the antennae of the apple blossom weevil. It has been suggested that perillene is one several terpene hydrocarbons in the emanation bouquet of apple tree buds which may be used by adult weevils as chemical cues to discrimination during host-searching behavior.
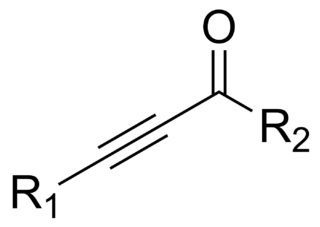
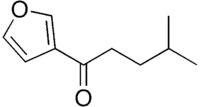
In organic chemistry, an ynone is an organic compound containing a ketone functional group and a C≡C triple bond. Many ynones are α,β-ynones, where the carbonyl and alkyne groups are conjugated. Capillin is a naturally occurring example. Some ynones are not conjugated.