| |
| Names | |
|---|---|
| Preferred IUPAC name (2Z,4E)-5-[(1R,5R,8S)-8-Hydroxy-1,5-dimethyl-3-oxo-6-oxabicyclo[3.2.1]octan-8-yl]-3-methylpenta-2,4-dienoic acid | |
| Other names Phaseic acid | |
| Identifiers | |
3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C15H20O5 | |
| Molar mass | 280.31 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
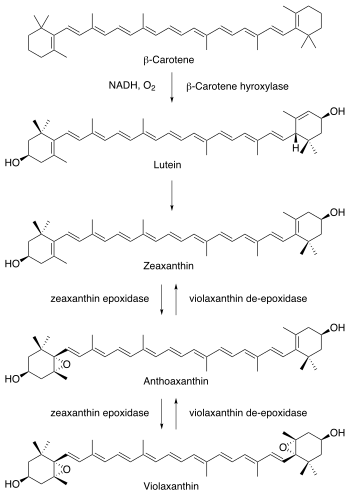
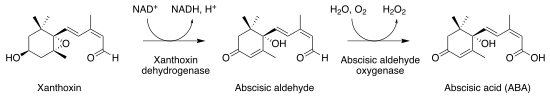
Phaseic acid is a terpenoid catabolite of abscisic acid. Like abscisic acid, it is a plant hormone associated with photosynthesis arrest [1] and abscission.