Ferrocene is an organometallic compound with the formula Fe(C
5H
5)
2. The molecule is a complex consisting of two cyclopentadienyl rings bound to a central iron atom. It is an orange solid with a camphor-like odor, that sublimes above room temperature, and is soluble in most organic solvents. It is remarkable for its stability: it is unaffected by air, water, strong bases, and can be heated to 400 °C without decomposition. In oxidizing conditions it can reversibly react with strong acids to form the ferrocenium cation Fe(C
5H
5)+
2.

A reagent is a substance or compound added to a system to cause a chemical reaction, or added to test if a reaction occurs. The terms reactant and reagent are often used interchangeably—however, a reactant is more specifically a substance consumed in the course of a chemical reaction. Solvents, though involved in the reaction mechanism, are usually not called reactants. Similarly, catalysts are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates.

Curcumin is a bright yellow chemical produced by plants of the Curcuma longa species. It is the principal curcuminoid of turmeric, a member of the ginger family, Zingiberaceae. It is sold as an herbal supplement, cosmetics ingredient, food flavoring, and food coloring.

In the fields of medicine, biotechnology and pharmacology, drug discovery is the process by which new candidate medications are discovered.

Lipinski's rule of five, also known as Pfizer's rule of five or simply the rule of five (RO5), is a rule of thumb to evaluate druglikeness or determine if a chemical compound with a certain pharmacological or biological activity has chemical properties and physical properties that would make it a likely orally active drug in humans. The rule was formulated by Christopher A. Lipinski in 1997, based on the observation that most orally administered drugs are relatively small and moderately lipophilic molecules.

Medicinal chemistry is discipline at the intersection of chemistry, especially synthetic organic chemistry, and pharmacology and various other biological specialties, where they are involved with design, chemical synthesis and development for market of pharmaceutical agents, or bio-active molecules (drugs).
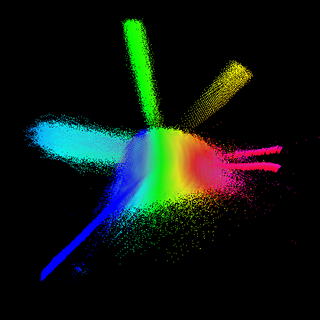
Chemical space is a concept in cheminformatics referring to the property space spanned by all possible molecules and chemical compounds adhering to a given set of construction principles and boundary conditions. It contains millions of compounds which are readily accessible and available to researchers. It is a library used in the method of molecular docking.

Isatin, also known as tribulin, is an organic compound derived from indole with formula C8H5NO2. The compound was first obtained by Otto Linné Erdman and Auguste Laurent in 1840 as a product from the oxidation of indigo dye by nitric acid and chromic acids.
Alexander Tropsha is a chemist and professor at the University of North Carolina - Chapel Hill. Tropsha is Associate Dean for Pharmacoinformatics and Data Science at the UNC Eshelman School of Pharmacy. His primary fields of research are cheminformatics and quantitative structure-activity relationship (QSAR) modeling in the context of drug discovery. As of 2015, Tropsha has been an associate editor of the American Chemical Society’s Journal of Chemical Information and Modeling.

Wilhelm Marceli Nencki was a Polish chemist and doctor.
Hit to lead (H2L) also known as lead generation is a stage in early drug discovery where small molecule hits from a high throughput screen (HTS) are evaluated and undergo limited optimization to identify promising lead compounds. These lead compounds undergo more extensive optimization in a subsequent step of drug discovery called lead optimization (LO). The drug discovery process generally follows the following path that includes a hit to lead stage:

Chemical similarity refers to the similarity of chemical elements, molecules or chemical compounds with respect to either structural or functional qualities, i.e. the effect that the chemical compound has on reaction partners in inorganic or biological settings. Biological effects and thus also similarity of effects are usually quantified using the biological activity of a compound. In general terms, function can be related to the chemical activity of compounds.
Inte:Ligand was founded in Maria Enzersdorf, Lower Austria (Niederösterreich) in 2003. They established the company headquarters on Mariahilferstrasse in Vienna, Austria that same year.

ChEMBL or ChEMBLdb is a manually curated chemical database of bioactive molecules with drug-like properties. It is maintained by the European Bioinformatics Institute (EBI), of the European Molecular Biology Laboratory (EMBL), based at the Wellcome Trust Genome Campus, Hinxton, UK.
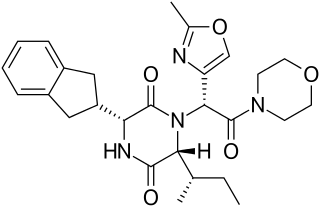
A diketopiperazine (DKP), also known as a dioxopiperazine or piperazinedione, is a class of organic compounds related to piperazine but with two amide linkages. Three regioisomers are possible, differing in the locations of the carbonyl groups.
Topological inhibitors are rigid three-dimensional molecules of inorganic, organic and hybrid compounds that form multicentered supramolecular interactions in vacant cavities of protein macromolecules and their complexes . Extensive surface and very diverse geometry make cage compounds with an encapsulated metal ion (clathrochelates) suitable for targeting both the active and allosteric sites of enzymes as well as the interfaces of their macromolecular complexes. An efficient structure- and concentration-dependent transcription inhibition in a model in vitro systems based on RNA and DNA polymerases by the iron(II) mono- and bis-clathrochelates at their submicro- and nanomolar concentrations, respectively, is observed in. Molecular docking and preincubation experiments suggested that these cage compounds form supramolecular assemblies with protein residues as well as with DNA and RNA. Thus, they are prospective precursors for the design of antiviral and anticancer drug candidates.
Matched molecular pair analysis (MMPA) is a method in cheminformatics that compares the properties of two molecules that differ only by a single chemical transformation, such as the substitution of a hydrogen atom by a chlorine one. Such pairs of compounds are known as matched molecular pairs (MMP). Because the structural difference between the two molecules is small, any experimentally observed change in a physical or biological property between the matched molecular pair can more easily be interpreted. The term was first coined by Kenny and Sadowski in the book Chemoinformatics in Drug Discovery.
Pan-assay interference compounds (PAINS) are chemical compounds that often give false positive results in high-throughput screens. PAINS tend to react nonspecifically with numerous biological targets rather than specifically affecting one desired target. A number of disruptive functional groups are shared by many PAINS.

Building block is a term in chemistry which is used to describe a virtual molecular fragment or a real chemical compound the molecules of which possess reactive functional groups. Building blocks are used for bottom-up modular assembly of molecular architectures: nano-particles, metal-organic frameworks, organic molecular constructs, supra-molecular complexes. Using building blocks ensures strict control of what a final compound or a (supra)molecular construct will be.
Jonathan Baell is an Australian medicinal chemist. He is a research professor in medicinal chemistry at the Monash Institute of Pharmaceutical Sciences (MIPS), the Director of the Australian Translational Medicinal Chemistry Facility and a Chief Investigator at the ARC Centre for Fragment-Based Design. He is also the President of the International Chemical Biology Society. His research focuses on the early stages of drug discovery, including high-throughput screening (HTS) library design, hit-to-lead and lead optimization for the treatment of a variety of diseases, such as malaria and neglected diseases.













