Melanin is a family of biomolecules organized as oligomers or polymers, which among other functions provide the pigments of many organisms. Melanin pigments are produced in a specialized group of cells known as melanocytes.

Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer of the skin's epidermis, the middle layer of the eye, the inner ear, vaginal epithelium, meninges, bones, and heart found in many mammals and birds. Melanin is a dark pigment primarily responsible for skin color. Once synthesized, melanin is contained in special organelles called melanosomes which can be transported to nearby keratinocytes to induce pigmentation. Thus darker skin tones have more melanosomes present than lighter skin tones. Functionally, melanin serves as protection against UV radiation. Melanocytes also have a role in the immune system.

Vitiligo is a chronic autoimmune disorder that causes patches of skin to lose pigment or color. The cause of vitiligo is unknown, but it may be related to immune system changes, genetic factors, stress, or sun exposure. Treatment options include topical medications, light therapy, surgery and cosmetics. The condition can show up on any skin type as a light peachy color and can appear on any place on the body in all sizes. The spots on the skin known as vitiligo are also able to “change” as spots lose and regain pigment; they will stay in relatively the same areas but can move over time and some big patches can move through the years but never disappear overnight.

Hypopigmentation is characterized specifically as an area of skin becoming lighter than the baseline skin color, but not completely devoid of pigment. This is not to be confused with depigmentation, which is characterized as the absence of all pigment. It is caused by melanocyte or melanin depletion, or a decrease in the amino acid tyrosine, which is used by melanocytes to make melanin. Some common genetic causes include mutations in the tyrosinase gene or OCA2 gene. As melanin pigments tend to be in the skin, eye, and hair, these are the commonly affected areas in those with hypopigmentation.
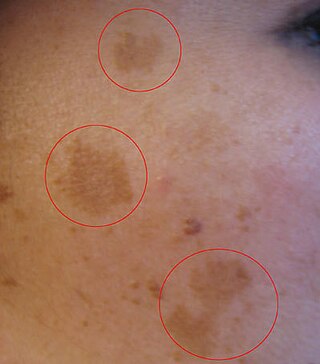
Melasma is a tan or dark skin discoloration. Melasma is thought to be caused by sun exposure, genetic predisposition, hormone changes, and skin irritation. Although it can affect anyone, it is particularly common in women, especially pregnant women and those who are taking oral or patch contraceptives or hormone replacement therapy medications.

Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper–Mason pathway. Firstly, the hydroxylation of a monophenol and secondly, the conversion of an o-diphenol to the corresponding o-quinone. o-Quinone undergoes several reactions to eventually form melanin. Tyrosinase is a copper-containing enzyme present in plant and animal tissues that catalyzes the production of melanin and other pigments from tyrosine by oxidation. It is found inside melanosomes which are synthesized in the skin melanocytes. In humans, the tyrosinase enzyme is encoded by the TYR gene.

Skin whitening, also known as skin lightening and skin bleaching, is the practice of using chemical substances in an attempt to lighten the skin or provide an even skin color by reducing the melanin concentration in the skin. Several chemicals have been shown to be effective in skin whitening, while some have proven to be toxic or have questionable safety profiles. This includes mercury compounds which may cause neurological problems and kidney problems.

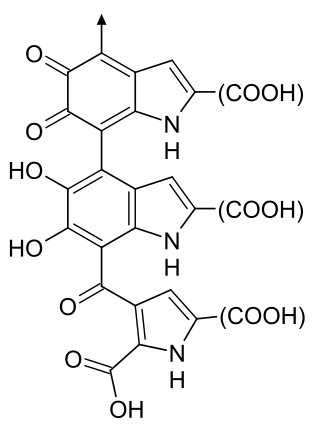
Citrinin is a mycotoxin which is often found in food. It is a secondary metabolite produced by fungi that contaminates long-stored food and it can cause a variety of toxic effects, including kidney, liver and cell damage. Citrinin is mainly found in stored grains, but sometimes also in fruits and other plant products.
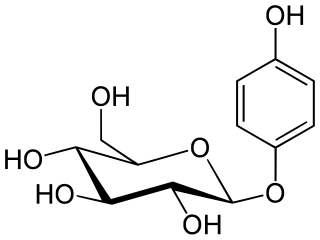
β-arbutin, also known by its International Nomenclature of Cosmetic Ingredients (INCI) name, arbutin, is a glycosylated derivative of hydroquinone. β-Arbutin is naturally present in the leaves and bark of a variety of plants, notably the bearberry plant, Arctostaphylos uva-ursi. Utilized as a biosynthetic active ingredient in topical treatments for skin lightening, β-arbutin is aimed at addressing hyperpigmentation issues. Its mechanism of action involves inhibiting the activity of tyrosinase, an essential enzyme for melanin synthesis in the human skin, thereby leading to a reduction in hyperpigmentation. It is important to distinguish β-arbutin from its structurally similar stereoisomer, α-arbutin, which exhibits similar effects in clinical applications.
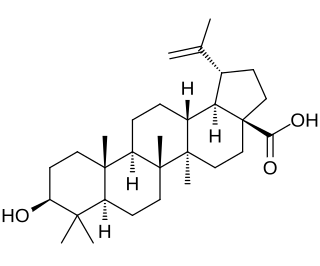
Betulinic acid is a naturally occurring pentacyclic triterpenoid which has antiretroviral, antimalarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase. It is found in the bark of several species of plants, principally the white birch from which it gets its name, same as the bracket fungus Fomitopsis betulina, but also the ber tree, selfheal, the tropical carnivorous plants Triphyophyllum peltatum and Ancistrocladus heyneanus, Diospyros leucomelas, a member of the persimmon family, Tetracera boiviniana, the jambul, flowering quince, rosemary, and Pulsatilla chinensis.
Polyphenol oxidase, an enzyme involved in fruit browning, is a tetramer that contains four atoms of copper per molecule.
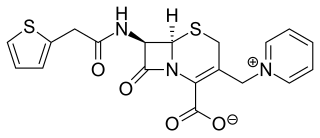
Cephaloridine is a first-generation semisynthetic derivative of antibiotic cephalosporin C. It is a Beta lactam antibiotic, like penicillin. Its chemical structure contains 3 cephems, 4 carboxyl groups and three pyridinium methyl groups.

Protocatechuic acid (PCA) is a dihydroxybenzoic acid, a type of phenolic acid. It is a major metabolite of antioxidant polyphenols found in green tea. It has mixed effects on normal and cancer cells in in vitro and in vivo studies. It is produced commercially from vanillin.
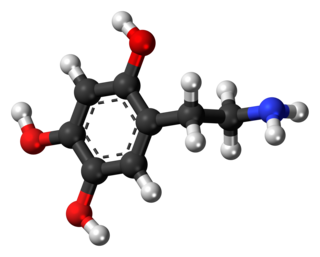
Oxidopamine, also known as 6-hydroxydopamine (6-OHDA) or 2,4,5-trihydroxyphenethylamine, is a synthetic monoaminergic neurotoxin used by researchers to selectively destroy dopaminergic and noradrenergic neurons in the brain.
Smoker's face describes the characteristic changes that happen to the faces of many people who smoke tobacco products. Smoking causes damage to the skin by depleting the skin of oxygen and nutrients. The general appearance is of accelerated ageing of the face, with a characteristic pattern of facial wrinkling and sallow coloration.
Postinflammatory hypopigmentation is a cutaneous condition characterized by decreased pigment in the skin following inflammation of the skin.
Arsenic biochemistry refers to biochemical processes that can use arsenic or its compounds, such as arsenate. Arsenic is a moderately abundant element in Earth's crust, and although many arsenic compounds are often considered highly toxic to most life, a wide variety of organoarsenic compounds are produced biologically and various organic and inorganic arsenic compounds are metabolized by numerous organisms. This pattern is general for other related elements, including selenium, which can exhibit both beneficial and deleterious effects. Arsenic biochemistry has become topical since many toxic arsenic compounds are found in some aquifers, potentially affecting many millions of people via biochemical processes.

HU-331 is a quinone anticarcinogenic drug synthesized from cannabidiol, a cannabinoid in the Cannabis sativa plant. It showed a great efficacy against oncogenic human cells. HU-331 does not cause arrest in cell cycle, cell apoptosis or caspase activation. HU-331 inhibits DNA topoisomerase II even at nanomolar concentrations, but has shown a negligible effect on the action of DNA topoisomerase I. The cannabinoid quinone HU-331 is a very specific inhibitor of topoisomerase II, compared with most known anticancer quinones. One of the main objectives of these studies is the development of a new quinone derived compound that produces anti-neoplastic activity while maintaining low toxicity at therapeutic doses.

Glyceryl octyl ascorbic acid (GO-VC) is an amphipathic derivative of vitamin C consisting of two ether linkages: a 1-octyl at position 2 and a glycerin at position 3. The chemical name is 2-glyceryl-3-octyl ascorbic acid. The isomer in which these two groups are swapped is also known.
The p-i concept refers to the pharmacological interaction of drugs with immune receptors. It explains a form of drug hypersensitivity, namely T cell stimulation, which can lead to various acute inflammatory manifestations such as exanthems, eosinophilia and systemic symptoms, Stevens–Johnson syndrome, toxic epidermal nercrolysis, and complications upon withdrawing the drug.

















