
Bluetongue disease is a noncontagious, insect-borne, viral disease of ruminants, mainly sheep and less frequently cattle, yaks, goats, buffalo, deer, dromedaries, and antelope. It is caused by Bluetongue virus (BTV). The virus is transmitted by the midges Culicoides imicola, Culicoides variipennis, and other culicoids.

Rift Valley fever (RVF) is a viral disease of humans and livestock that can cause mild to severe symptoms. The mild symptoms may include: fever, muscle pains, and headaches which often last for up to a week. The severe symptoms may include: loss of sight beginning three weeks after the infection, infections of the brain causing severe headaches and confusion, and bleeding together with liver problems which may occur within the first few days. Those who have bleeding have a chance of death as high as 50%.
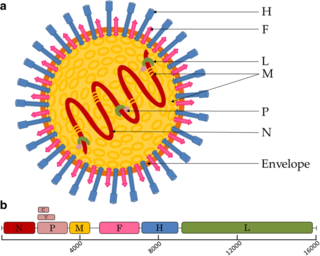
Paramyxoviridae is a family of negative-strand RNA viruses in the order Mononegavirales. Vertebrates serve as natural hosts. Diseases associated with this family include measles, mumps, and respiratory tract infections. The family has four subfamilies, 17 genera, three of which are unassigned to a subfamily, and 78 species.

Coronaviruses are a group of related RNA viruses that cause diseases in mammals and birds. In humans and birds, they cause respiratory tract infections that can range from mild to lethal. Mild illnesses in humans include some cases of the common cold, while more lethal varieties can cause SARS, MERS and COVID-19. In cows and pigs they cause diarrhea, while in mice they cause hepatitis and encephalomyelitis.

Bunyavirales is an order of segmented negative-strand RNA viruses with mainly tripartite genomes. Member viruses infect arthropods, plants, protozoans, and vertebrates. It is the only order in the class Ellioviricetes. The name Bunyavirales derives from Bunyamwera, where the original type species Bunyamwera orthobunyavirus was first discovered. Ellioviricetes is named in honor of late virologist Richard M. Elliott for his early work on bunyaviruses.
Bwamba orthobunyavirus (BWAV) belongs to the genus Orthobunyavirus and the order Bunyavirales RNA viruses. BWAV is present in large parts of Africa, endemic in Mozambique, Tanzania and Uganda. It is transmitted to humans through mosquito bites and results in a brief benign generalised infection with headache, skin rash, diarrhea and joint pain and lasts 4–5 days. The animal reservoir of the virus includes birds, monkeys and donkeys.

Oropouche fever is a tropical viral infection transmitted by biting midges and mosquitoes from the blood of sloths to humans. This disease is named after the region where it was first discovered and isolated at the Trinidad Regional Virus Laboratory in 1955 by the Oropouche River in Trinidad and Tobago. Oropouche fever is caused by a specific arbovirus, the Oropouche virus (OROV), of the Bunyaviridae family.

Orbivirus is a genus of double-stranded RNA viruses in the family Reoviridae and subfamily Sedoreovirinae. Unlike other reoviruses, orbiviruses are arboviruses. They can infect and replicate within a wide range of arthropod and vertebrate hosts. Orbiviruses are named after their characteristic doughnut-shaped capsomers.

Aphthovirus is a viral genus of the family Picornaviridae. Aphthoviruses infect split-hooved animals, and include the causative agent of foot-and-mouth disease, Foot-and-mouth disease virus (FMDV). There are seven FMDV serotypes: A, O, C, SAT 1, SAT 2, SAT 3 and Asia 1, and four non-FMDV serotypes belonging to three additional species Bovine rhinitis A virus (BRAV), Bovine rhinitis B virus (BRBV) and Equine rhinitis A virus (ERAV).
Pestivirus is a genus of viruses, in the family Flaviviridae. Viruses in the genus Pestivirus infect mammals, including members of the family Bovidae and the family Suidae. There are 11 species in this genus. Diseases associated with this genus include: hemorrhagic syndromes, abortion, and fatal mucosal disease.

Orthobunyavirus is a genus of the Peribunyaviridae family in the order Bunyavirales. There are currently ~170 viruses recognised in this genus. These have been assembled into 103 species and 20 serogroups.

Akabane virus is an insect-transmitted virus that causes congenital abnormalities of the central nervous systems in ruminants. The virus is found in Australia, where it is most commonly spread by biting midges of the Culicoides species.

Double-stranded RNA viruses are a polyphyletic group of viruses that have double-stranded genomes made of ribonucleic acid. The double-stranded genome is used as a template by the viral RNA-dependent RNA polymerase (RdRp) to transcribe a positive-strand RNA functioning as messenger RNA (mRNA) for the host cell's ribosomes, which translate it into viral proteins. The positive-strand RNA can also be replicated by the RdRp to create a new double-stranded viral genome.

Torovirus is a genus of enveloped, positive-strand RNA viruses in the order Nidovirales and family Tobaniviridae. They primarily infect vertebrates, especially cattle, pigs, and horses. Diseases associated with this genus include gastroenteritis, which commonly presents in mammals. Torovirus is the only genus in the monotypic subfamily Torovirinae. Torovirus is also a monotypic taxon, containing only one subgenus, Renitovirus.
Bunyamwera orthobunyavirus (BUNV) is a negative-sense, single-stranded enveloped RNA virus. It is assigned to the Orthobunyavirus genus, in the Bunyavirales order.
Bovine coronavirus is a coronavirus which is a member of the species Betacoronavirus 1. The infecting virus is an enveloped, positive-sense, single-stranded RNA virus which enters its host cell by binding to the N-acetyl-9-O-acetylneuraminic acid recepter. Infection causes calf enteritis and contributes to the enzootic pneumonia complex in calves. It can also cause winter dysentery in adult cattle. It can infect both domestic and wild ruminants and has a worldwide distribution. Transmission is horizontal, via oro-fecal or respiratory routes. Like other coronaviruses from genus Betacoronavirus, subgenus Embecovirus, it has a surface protein called hemagglutinin esterase (HE) in addition to the four structural proteins shared by all coronaviruses.
In 1954 the Hazara orthonairovirus, one of the 34 tick-borne viruses of the genus Orthonairovirus, was discovered in Pakistan in the Ixodes tick native to that region. Today this virus is studied in mice in an attempt to develop treatments for the highly pathogenic Crimean-Congo Hemorrhagic Fever virus.
Batai orthobunyavirus (BATV) is a RNA virus belonging to order Bunyavirales, genus Orthobunyavirus.
Cache Valley orthobunyavirus (CVV) is a member of the order Bunyavirales, genus Orthobunyavirus, and serogroup Bunyamwera, which was first isolated in 1956 from Culiseta inornata mosquitos collected in Utah's Cache Valley. CVV is an enveloped arbovirus, nominally 80–120 nm in diameter, whose genome is composed of three single-stranded, negative-sense RNA segments. The large segment of related bunyaviruses is approximately 6800 bases in length and encodes a probable viral polymerase. The middle CVV segment has a 4463-nucleotide sequence and the smallest segment encodes for the nucleocapsid, and a second non-structural protein. CVV has been known to cause outbreaks of spontaneous abortion and congenital malformations in ruminants such as sheep and cattle. CVV rarely infects humans, but when they are infected it has caused encephalitis and multiorgan failure.
Jamestown Canyon encephalitis is an infectious disease caused by the Jamestown Canyon virus, an orthobunyavirus of the California serogroup. It is mainly spread during the summer by different mosquito species in the United States and Canada.
















