Nylon is a generic designation for a family of synthetic polymers composed of polyamides. Nylon is a silk-like thermoplastic, generally made from petroleum, that can be melt-processed into fibers, films, or shapes. Nylon polymers can be mixed with a wide variety of additives to achieve many different property variations. Nylon polymers have found significant commercial applications in fabric and fibers, in shapes, and in films.
A polyamide is a polymer with repeating units linked by amide bonds.
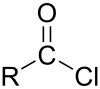
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.

An acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
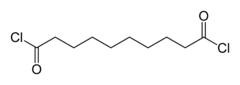
Adipoyl chloride (or adipoyl dichloride) is the organic compound with the formula (CH2CH2C(O)Cl)2. It is a colorless liquid. It reacts with water to give adipic acid.

Oxalyl chloride is an organic chemical compound with the formula (COCl)2. This colorless, sharp-smelling liquid, the diacyl chloride of oxalic acid, is a useful reagent in organic synthesis.

Thionyl chloride is an inorganic compound with the chemical formula SOCl
2. It is a moderately volatile colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a chlorinating reagent, with approximately 45,000 tonnes per year being produced during the early 1990s, but is occasionally also used as a solvent. It is toxic, reacts with water, and is also listed under the Chemical Weapons Convention as it may be used for the production of chemical weapons.

Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C7H5ClO. It is a colourless, fuming liquid with an irritating odour. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins.
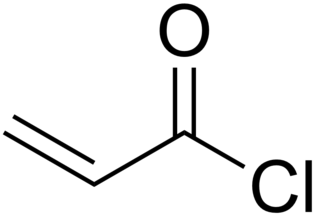
Acryloyl chloride, also known as 2-propenoyl chloride or acrylic acid chloride, is the organic compound with the formula CH2=CHCO(Cl). It is a colorless liquid, although aged samples appear yellow. Although it belongs to the acid chlorides group of compounds, its synthesis and reactivity are somewhat different.

The thionyl group is SO, a sulfur atom plus an oxygen atom.

Sulfuryl chloride is an inorganic compound with the formula SO2Cl2. At room temperature, it is a colorless liquid with a pungent odor. Sulfuryl chloride is not found in nature, as can be inferred from its rapid hydrolysis.

Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing.

Thionyl bromide is the chemical compound SOBr2. It is less stable and less widely used than its chloride analogue, thionyl chloride. It is prepared by the action of hydrogen bromide on thionyl chloride, a characteristic reaction where a stronger acid is converted to a weaker acid:
Sulfonyl halide groups occur when a sulfonyl functional group is singly bonded to a halogen atom. They have the general formula RSO2X where X is a halogen. The stability of sulfonyl halides decreases in the order fluorides > chlorides > bromides > iodides, all four types being well known. The sulfonyl chlorides and fluorides are of dominant importance in this series.

Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.

Oxazoline is a five-membered heterocyclic chemical compound containing one atom each of oxygen and nitrogen. It was likely first synthesized in 1884 but it was not until 5 years later that Siegmund Gabriel correctly assigned the structure. It was named in-line with the Hantzsch–Widman nomenclature and is part of a family of heterocyclic compounds, where it exists between oxazole and oxazolidine in terms of saturation.
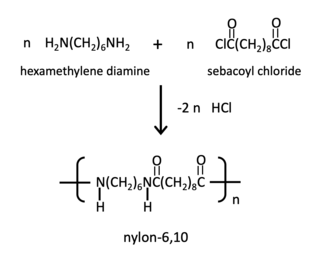
The nylon rope trick is a scientific demonstration that illustrates some of the fundamental chemical principles of step-growth polymerization and provides students and other observers with a hands-on demonstration of the preparation of a synthetic polymer.

Dimethylcarbamoyl chloride (DMCC) is a reagent for transferring a dimethylcarbamoyl group to alcoholic or phenolic hydroxyl groups forming dimethyl carbamates, usually having pharmacological or pesticidal activities. Because of its high toxicity and its carcinogenic properties shown in animal experiments and presumably also in humans, dimethylcarbamoyl chloride can only be used under stringent safety precautions.
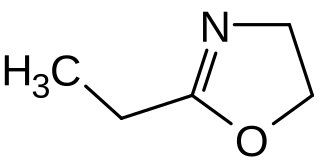
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications.
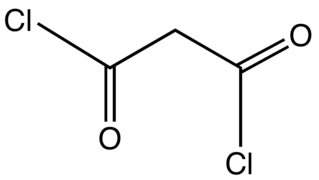
Malonyl chloride is the organic compound with the formula CH2(COCl)2. It is the acyl chloride derivative of malonic acid. It is a colorless liquid although samples are often deeply colored owing to impurities. The compound degrades at room temperature after a few days. It used as a reagent in organic synthesis.