Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Pauling scale, behind only oxygen and fluorine.

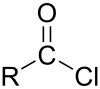
In organic chemistry, an acyl halide is a chemical compound derived from an oxoacid by replacing a hydroxyl group with a halide group.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

In chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.

Sodium chlorate is an inorganic compound with the chemical formula NaClO3. It is a white crystalline powder that is readily soluble in water. It is hygroscopic. It decomposes above 300 °C to release oxygen and leaves sodium chloride. Several hundred million tons are produced annually, mainly for applications in bleaching pulp to produce high brightness paper.

Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions.

Cadmium chloride is a white crystalline compound of cadmium and chloride, with the formula CdCl2. This salt is a hygroscopic solid that is highly soluble in water and slightly soluble in alcohol. The crystal structure of cadmium chloride (described below), is a reference for describing other crystal structures. Also known are CdCl2•H2O and CdCl2•5H2O.

Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution. The osmolarity of a solution is usually expressed as Osm/L, in the same way that the molarity of a solution is expressed as "M". Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of osmoles of solute particles per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration.
Benzyl bromide is an organic compound with the formula C6H5CH2Br. The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties. The compound is a reagent for introducing benzyl groups.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.

Rubidium chloride is the chemical compound with the formula RbCl. This alkali metal halide salt is composed of rubidium and chlorine, and finds diverse uses ranging from electrochemistry to molecular biology.
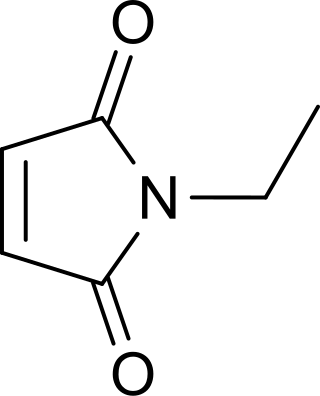
N-Ethylmaleimide (NEM) is an organic compound that is derived from maleic acid. It contains the amide functional group, but more importantly it is an alkene that is reactive toward thiols and is commonly used to modify cysteine residues in proteins and peptides.

Methyl vinyl ketone (MVK, IUPAC name: butenone) is the organic compound with the formula CH3C(O)CH=CH2. It is a reactive compound classified as an enone, in fact the simplest example thereof. It is a colorless, flammable, highly toxic liquid with a pungent odor. It is soluble in water and polar organic solvents. It is a useful intermediate in the synthesis of other compounds.

Trospium chloride is used to treat overactive bladder.

Mercuric amidochloride is an inorganic compound with the formula Hg(NH2)Cl.

Cromakalim (INN) is a potassium channel-opening vasodilator. The active isomer is levcromakalim. It acts on ATP-sensitive potassium channels and so causes membrane hyperpolarization. It can be used to treat hypertension as it will relax vascular smooth muscle to lower blood pressure. Hyperpolarisation of smooth muscle cell membranes pulls their membrane potential away from the threshold, so making it more difficult to excite them and thereby cause relaxation.

A channel blocker is the biological mechanism in which a particular molecule is used to prevent the opening of ion channels in order to produce a physiological response in a cell. Channel blocking is conducted by different types of molecules, such as cations, anions, amino acids, and other chemicals. These blockers act as ion channel antagonists, preventing the response that is normally provided by the opening of the channel.

The Quelet reaction is an organic coupling reaction in which a phenolic ether reacts with an aliphatic aldehyde to generate an α-chloroalkyl derivative. The Quelet reaction is an example of a larger class of reaction, electrophilic aromatic substitution. The reaction is named after its creator R. Quelet, who first reported the reaction in 1932, and is similar to the Blanc chloromethylation process.

In chemistry, congeners are chemical substances "related to each other by origin, structure, or function".

Trifluoroacetyl chloride (also known as TFAC) is a toxic gaseous chemical compound with the chemical formula C2ClF3O. TFAC is the perfluorinated version of acetyl chloride. The compound is a gas, but it is usually shipped as a liquid under high pressure.