Related Research Articles

An extremophile is an organism that is able to live in extreme environments, i.e. environments with conditions approaching or expanding the limits of what known life can adapt to, such as extreme temperature, radiation, salinity, or pH level.
A hyperthermophile is an organism that thrives in extremely hot environments—from 60 °C (140 °F) upwards. An optimal temperature for the existence of hyperthermophiles is often above 80 °C (176 °F). Hyperthermophiles are often within the domain Archaea, although some bacteria are also able to tolerate extreme temperatures. Some of these bacteria are able to live at temperatures greater than 100 °C, deep in the ocean where high pressures increase the boiling point of water. Many hyperthermophiles are also able to withstand other environmental extremes, such as high acidity or high radiation levels. Hyperthermophiles are a subset of extremophiles. Their existence may support the possibility of extraterrestrial life, showing that life can thrive in environmental extremes.
Alkaliphiles are a class of extremophilic microbes capable of survival in alkaline environments, growing optimally around a pH of 10. These bacteria can be further categorized as obligate alkaliphiles, facultative alkaliphiles and haloalkaliphiles.

Kaikō was a remotely operated underwater vehicle (ROV) built by the Japan Agency for Marine-Earth Science and Technology (JAMSTEC) for exploration of the deep sea. Kaikō was the second of only five vessels ever to reach the bottom of the Challenger Deep, as of 2019. Between 1995 and 2003, this 10.6 ton unmanned submersible conducted more than 250 dives, collecting 350 biological species, some of which could prove to be useful in medical and industrial applications. On 29 May 2003, Kaikō was lost at sea off the coast of Shikoku Island during Typhoon Chan-Hom, when a secondary cable connecting it to its launcher at the ocean surface broke.
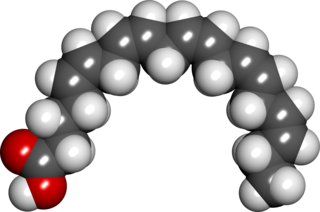
Eicosapentaenoic acid is an omega-3 fatty acid. In physiological literature, it is given the name 20:5(n-3). It also has the trivial name timnodonic acid. In chemical structure, EPA is a carboxylic acid with a 20-carbon chain and five cis double bonds; the first double bond is located at the third carbon from the omega end.

A thermoacidophile is an extremophilic microorganism that is both thermophilic and acidophilic; i.e., it can grow under conditions of high temperature and low pH. The large majority of thermoacidophiles are archaea or bacteria, though occasional eukaryotic examples have been reported. Thermoacidophiles can be found in hot springs and solfataric environments, within deep sea vents, or in other environments of geothermal activity. They also occur in polluted environments, such as in acid mine drainage.
A piezophile is an organism with optimal growth under high hydrostatic pressure i.e. an organism that has its maximum rate of growth at a hydrostatic pressure equal to or above 10 MPa, when tested over all permissible temperatures. Originally, the term barophile was used for these organisms, but since the prefix "baro-" stands for weight, the term piezophile was given preference. Like all definitions of extremophiles, the definition of piezophiles is anthropocentric, and humans consider that moderate values for hydrostatic pressure are those around 1 atm. Hyperpiezophiles are organisms that have their maximum growth rate above 50 MPa.
Sulfur-reducing bacteria are microorganisms able to reduce elemental sulfur (S0) to hydrogen sulfide (H2S). These microbes use inorganic sulfur compounds as electron acceptors to sustain several activities such as respiration, conserving energy and growth, in absence of oxygen. The final product of these processes, sulfide, has a considerable influence on the chemistry of the environment and, in addition, is used as electron donor for a large variety of microbial metabolisms. Several types of bacteria and many non-methanogenic archaea can reduce sulfur. Microbial sulfur reduction was already shown in early studies, which highlighted the first proof of S0 reduction in a vibrioid bacterium from mud, with sulfur as electron acceptor and H
2 as electron donor. The first pure cultured species of sulfur-reducing bacteria, Desulfuromonas acetoxidans, was discovered in 1976 and described by Pfennig Norbert and Biebel Hanno as an anaerobic sulfur-reducing and acetate-oxidizing bacterium, not able to reduce sulfate. Only few taxa are true sulfur-reducing bacteria, using sulfur reduction as the only or main catabolic reaction. Normally, they couple this reaction with the oxidation of acetate, succinate or other organic compounds. In general, sulfate-reducing bacteria are able to use both sulfate and elemental sulfur as electron acceptors. Thanks to its abundancy and thermodynamic stability, sulfate is the most studied electron acceptor for anaerobic respiration that involves sulfur compounds. Elemental sulfur, however, is very abundant and important, especially in deep-sea hydrothermal vents, hot springs and other extreme environments, making its isolation more difficult. Some bacteria – such as Proteus, Campylobacter, Pseudomonas and Salmonella – have the ability to reduce sulfur, but can also use oxygen and other terminal electron acceptors.

Shewanella is the sole genus included in the marine bacteria family Shewanellaceae. Some species within it were formerly classed as Alteromonas. Shewanella consists of facultatively anaerobic Gram-negative rods, most of which are found in extreme aquatic habitats where the temperature is very low and the pressure is very high. Shewanella bacteria are a normal component of the surface flora of fish and are implicated in fish spoilage. Shewanella chilikensis, a species of the genus Shewanella commonly found in the marine sponges of Saint Martin's Island of the Bay of Bengal, Bangladesh.

Photobacterium profundum is a deep sea Gammaproteobacterium, belonging to the family Vibrionaceae and genus Photobacterium. Like other members of this genus, P. profundum is a marine organism and has two circular chromosomes. P. profundum is a gram-negative rod with the ability for growth at temperatures from 0 °C to 25 °C and pressures from 0.1 MPa to 70 MPa depending on the strain. It has a requirement for salt, is able to metabolise a wide range of simple and complex carbohydrates and has two flagella systems. Cells are rod shape, 2-4μm long and 0.8-1.0μm wide, with a single unsheathed flagella. This bacterium was originally isolated in 1986 from the Sulu Sea and there are currently 4 cultured wild-type strains of P. profundum,.

Cytochrome P450 2S1 is a protein that in humans is encoded by the CYP2S1 gene. The gene is located in chromosome 19q13.2 within a cluster including other CYP2 family members such as CYP2A6, CYP2A13, CYP2B6, and CYP2F1.

Cytochrome P450 4F12 is a protein that in humans is encoded by the CYP4F12 gene.

Shewanella oneidensis is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name.
Epoxygenases are a set of membrane-bound, heme-containing cytochrome P450 enzymes that metabolize polyunsaturated fatty acids to epoxide products that have a range of biological activities. The most thoroughly studied substrate of the CYP epoxylgenases is arachidonic acid. This polyunsaturated fatty acid is metabolized by cyclooxygenases to various prostaglandin, thromboxane, and prostacyclin metabolites in what has been termed the first pathway of eicosanoid production; it is also metabolized by various lipoxygenases to hydroxyeicosatetraenoic acids and leukotrienes in what has been termed the second pathway of eicosanoid production. The metabolism of arachidonic acid to epoxyeicosatrienoic acids by the CYP epoxygenases has been termed the third pathway of eicosanoid metabolism. Like the first two pathways of eicosanoid production, this third pathway acts as a signaling pathway wherein a set of enzymes metabolize arachidonic acid to a set of products that act as secondary signals to work in activating their parent or nearby cells and thereby orchestrate functional responses. However, none of these three pathways is limited to metabolizing arachidonic acid to eicosanoids. Rather, they also metabolize other polyunsaturated fatty acids to products that are structurally analogous to the eicosanoids but often have different bioactivity profiles. This is particularly true for the CYP epoxygenases which in general act on a broader range of polyunsaturated fatty acids to form a broader range of metabolites than the first and second pathways of eicosanoid production. Furthermore, the latter pathways form metabolites many of which act on cells by binding with and thereby activating specific and well-characterized receptor proteins; no such receptors have been fully characterized for the epoxide metabolites. Finally, there are relatively few metabolite-forming lipoxygenases and cyclooxygenases in the first and second pathways and these oxygenase enzymes share similarity between humans and other mammalian animal models. The third pathway consists of a large number of metabolite-forming CYP epoxygenases and the human epoxygenases have important differences from those of animal models. Partly because of these differences, it has been difficult to define clear roles for the epoxygenase-epoxide pathways in human physiology and pathology.
Shewanella baltica DSS12 is a gram-negative bacterium. Its type strain is NCTC 10735. It is of particular importance in fish spoilage.
Shewanella livingstonensis is a species of bacteria. Its cells are psychrophilic, gram-negative, rod-shaped, facultatively anaerobic and motile by means of a single polar flagellum. Its type strain is LMG 19866T.
Psychroflexus torquis is a species of bacterium. It is psychrophilic and its type strain is strain ACAM 623T. It has the ability to synthesize the polyunsaturated fatty acids eicosapentaenoic acid and arachidonic acid.
Thermococcus barophilus is a piezophilic and hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. It is anaerobic and sulfur-metabolising, with type strain MPT.
Hentriacontanonaene is a long-chain polyunsaturated hydrocarbon produced by numerous gamma-proteobacteria primarily from the marine environment. Hentriacontanonaene was originally isolated from bacterial isolates from Antarctic sea ice cores. All isolated bacteria that produced hentriacontanonaene also produced the polyunsaturated fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Given its polyunsaturated nature it has been proposed that this molecule is produced as part of a response to maintain optimal membrane fluidity.

Epoxyeicosatetraenoic acids are a set of biologically active epoxides that various cell types make by metabolizing the omega 3 fatty acid, eicosapentaenoic acid (EPA), with certain cytochrome P450 epoxygenases. These epoxygenases can metabolize EPA to as many as 10 epoxides that differ in the site and/or stereoisomer of the epoxide formed; however, the formed EEQs, while differing in potency, often have similar bioactivities and are commonly considered together.
References
- 1 2 3 Nogi, Y., C. Kato, and K. Horikoshi. "Taxonomic studies of deep-sea barophilic Shewanella strains and description of Shewanella violacea sp. nov." Archives of Microbiology170(5) (1998): 331–38. Print.
- ↑ H. Kobayashi, Y. Nogi, and K. Horikoshi. "New violet 3,3'-bipyridyl pigment purified from deep-sea microorganism Shewanella violacea DSS12" Extremophiles, 11 (2007): 245–250. Print.
- 1 2 Chikuma, Sayaka, Ryota Kasahara, Chiaki Kato, and Hideyuki Tamegai. "Bacterial adaptation to high pressure: a respiratory system in the deep-sea bacterium Shewanella violacea DSS12." FEMS Microbiology Letters267(1) (2007): 108–12. Print.
- 1 2 3 4 5 Kato, Chiaki, and Yuichi Nogi. "Correlation between phylogenetic structure and function: examples from deep-sea Shewanella." FEMS Microbiology Ecology35(3) (2001): 223–30. Print.
- 1 2 Aono, Eiji et al. "Complete genome sequence and comparative analysis of Shewanella violacea, a psychrophilic and piezophilic bacterium from deep sea floor sediments." Molecular BioSystems6 (2010): 1216–226. Print.
- ↑ Kawamoto, Jun et al. "Favourable effects of eicosapentaenoic acid on the late step of the cell division in a piezophilic bacterium, Shewanella violacea DSS12, at high-hydrostatic pressures." Environmental Microbiology13(8) (2011): 2293-298. Print.
- ↑ Tamegai, H. "Piezotolerance of the respiratory terminal oxidase activity of the piezophilic Shewanella violacea DSS12 as compared to non-piezophilic species." Biosci. Biotechnol. Biochem.75(5) (2011): 919–24. Print
- ↑ Kawamoto, J., T. Kurihara, K. Yamamoto, M. Nagayasu, Y. Tani, H. Mihara, M. Hosokawa, T. Baba, S. B. Sato, and N. Esaki. "Eicosapentaenoic acid plays a beneficial role in membrane organization and cell division of a cold-adapted bacterium, Shewanella livingstonensis Ac10." Journal of Bacteriology191(2) (2009): 632–40. Print.
- ↑ Nakasone, Kaoru. "Piezoregulation of cytochrome bd biosynthesis in deep-sea bacterium Shewanella violacea DSS12." Research Reports of the School of Engineering39 (2005): 11–14. Print.