In neuroscience, an F wave is one of several motor responses which may follow the direct motor response (M) evoked by electrical stimulation of peripheral motor or mixed nerves. F-waves are the second of two late voltage changes observed after stimulation is applied to the skin surface above the distal region of a nerve, in addition to the H-reflex which is a muscle reaction in response to electrical stimulation of innervating sensory fibers. Traversal of F-waves along the entire length of peripheral nerves between the spinal cord and muscle, allows for assessment of motor nerve conduction between distal stimulation sites in the arm and leg, and related motoneurons (MN's) in the cervical and lumbosacral cord. F-waves are able to assess both afferent and efferent loops of the alpha motor neuron in its entirety. As such, various properties of F-wave motor nerve conduction are analyzed in nerve conduction studies (NCS), and often used to assess polyneuropathies, resulting from states of neuronal demyelination and loss of peripheral axonal integrity.
In physiology, nociception, also nocioception; from Latin nocere 'to harm/hurt') is the sensory nervous system's process of encoding noxious stimuli. It deals with a series of events and processes required for an organism to receive a painful stimulus, convert it to a molecular signal, and recognize and characterize the signal to trigger an appropriate defensive response.
Clinical neurophysiology is a medical specialty that studies the central and peripheral nervous systems through the recording of bioelectrical activity, whether spontaneous or stimulated. It encompasses both research regarding the pathophysiology along with clinical methods used to diagnose diseases involving both central and peripheral nervous systems. Examinations in the clinical neurophysiology field are not limited to tests conducted in a laboratory. It is thought of as an extension of a neurologic consultation. Tests that are conducted are concerned with measuring the electrical functions of the brain, spinal cord, and nerves in the limbs and muscles. It can give the precise definition of site, the type and degree of the lesion, along with revealing the abnormalities that are in question. Due to these abilities, clinical neurophysiology is used to mainly help diagnose diseases rather than treat them.
An evoked potential or evoked response is an electrical potential in a specific pattern recorded from a specific part of the nervous system, especially the brain, of a human or other animals following presentation of a stimulus such as a light flash or a pure tone. Different types of potentials result from stimuli of different modalities and types. Evoked potential is distinct from spontaneous potentials as detected by electroencephalography (EEG), electromyography (EMG), or other electrophysiologic recording method. Such potentials are useful for electrodiagnosis and monitoring that include detections of disease and drug-related sensory dysfunction and intraoperative monitoring of sensory pathway integrity.
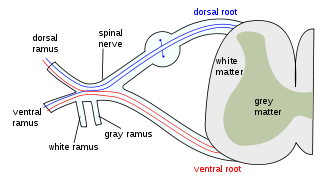
A spinal nerve is a mixed nerve, which carries motor, sensory, and autonomic signals between the spinal cord and the body. In the human body there are 31 pairs of spinal nerves, one on each side of the vertebral column. These are grouped into the corresponding cervical, thoracic, lumbar, sacral and coccygeal regions of the spine. There are eight pairs of cervical nerves, twelve pairs of thoracic nerves, five pairs of lumbar nerves, five pairs of sacral nerves, and one pair of coccygeal nerves. The spinal nerves are part of the peripheral nervous system.

In neuroanatomy, the trigeminal nerve (lit. triplet nerve), also known as the fifth cranial nerve, cranial nerve V, or simply CN V, is a cranial nerve responsible for sensation in the face and motor functions such as biting and chewing; it is the most complex of the cranial nerves. Its name (trigeminal, from Latin tri- 'three' and -geminus 'twin') derives from each of the two nerves (one on each side of the pons) having three major branches: the ophthalmic nerve (V1), the maxillary nerve (V2), and the mandibular nerve (V3). The ophthalmic and maxillary nerves are purely sensory, whereas the mandibular nerve supplies motor as well as sensory (or "cutaneous") functions. Adding to the complexity of this nerve is that autonomic nerve fibers as well as special sensory fibers (taste) are contained within it.

The grey columns are three regions of the somewhat ridge-shaped mass of grey matter in the spinal cord. These regions present as three columns: the anterior grey column, the posterior grey column, and the lateral grey column, all of which are visible in cross-section of the spinal cord.

The pyramidal tracts include both the corticobulbar tract and the corticospinal tract. These are aggregations of efferent nerve fibers from the upper motor neurons that travel from the cerebral cortex and terminate either in the brainstem (corticobulbar) or spinal cord (corticospinal) and are involved in the control of motor functions of the body.

The spinothalamic tract is a nerve tract in the anterolateral system in the spinal cord. This tract is an ascending sensory pathway to the thalamus. From the ventral posterolateral nucleus in the thalamus, sensory information is relayed upward to the somatosensory cortex of the postcentral gyrus.

The dorsal column–medial lemniscus pathway (DCML) (also known as the posterior column-medial lemniscus pathway is the major sensory pathway of the central nervous system that conveys sensations of fine touch, vibration, two-point discrimination, and proprioception from the skin and joints. It transmits this information to the somatosensory cortex of the postcentral gyrus in the parietal lobe of the brain. The pathway receives information from sensory receptors throughout the body, and carries this in the gracile fasciculus and the cuneate fasciculus, tracts that make up the white matter dorsal columns of the spinal cord. At the level of the medulla oblongata, the fibers of the tracts decussate and are continued in the medial lemniscus, on to the thalamus and relayed from there through the internal capsule and transmitted to the somatosensory cortex. The name dorsal-column medial lemniscus comes from the two structures that carry the sensory information: the dorsal columns of the spinal cord, and the medial lemniscus in the brainstem.
Neurapraxia is a disorder of the peripheral nervous system in which there is a temporary loss of motor and sensory function due to blockage of nerve conduction, usually lasting an average of six to eight weeks before full recovery. Neurapraxia is derived from the word apraxia, meaning “loss or impairment of the ability to execute complex coordinated movements without muscular or sensory impairment”.

A nerve conduction study (NCS) is a medical diagnostic test commonly used to evaluate the function, especially the ability of electrical conduction, of the motor and sensory nerves of the human body. These tests may be performed by medical specialists such as clinical neurophysiologists, physical therapists, physiatrists, and neurologists who subspecialize in electrodiagnostic medicine. In the United States, neurologists and physiatrists receive training in electrodiagnostic medicine (performing needle electromyography as part of residency training and, in some cases, acquire additional expertise during a fellowship in clinical neurophysiology, electrodiagnostic medicine, or neuromuscular medicine. Outside the US, clinical neurophysiologists learn needle EMG and NCS testing.

The human secondary somatosensory cortex is a region of sensory cortex in the parietal operculum on the ceiling of the lateral sulcus.

Monoplegia is paralysis of a single limb, usually an arm. Common symptoms associated with monoplegic patients are weakness, numbness, and pain in the affected limb. Monoplegia is a type of paralysis that falls under hemiplegia. While hemiplegia is paralysis of half of the body, monoplegia is localized to a single limb or to a specific region of the body. Monoplegia of the upper limb is sometimes referred to as brachial monoplegia, and that of the lower limb is called crural monoplegia. Monoplegia in the lower extremities is not as common of an occurrence as in the upper extremities. Monoparesis is a similar, but less severe, condition because one limb is very weak, not paralyzed. For more information, see paresis.

The vestibulospinal tract is a nerve tract in the central nervous system. Specifically, it is a component of the extrapyramidal system and is classified as a component of the medial pathway. Like other descending motor pathways, the vestibulospinal fibers of the tract relay information from nuclei to motor neurons. The vestibular nuclei receive information through the vestibulocochlear nerve about changes in the orientation of the head. The nuclei relay motor commands through the vestibulospinal tract. The function of these motor commands is to alter muscle tone, extend, and change the position of the limbs and head with the goal of supporting posture and maintaining balance of the body and head.

The posterolateral tract is a small strand situated in relation to the tip of the posterior column close to the entrance of the posterior nerve roots. It is present throughout the spinal cord, and is most developed in the upper cervical regions.

Group C nerve fibers are one of three classes of nerve fiber in the central nervous system (CNS) and peripheral nervous system (PNS). The C group fibers are unmyelinated and have a small diameter and low conduction velocity, whereas Groups A and B are myelinated. Group C fibers include postganglionic fibers in the autonomic nervous system (ANS), and nerve fibers at the dorsal roots. These fibers carry sensory information.
Pallesthesia, or vibratory sensation, is the ability to perceive vibration. This sensation, often conducted through skin and bone, is usually generated by mechanoreceptors such as Pacinian corpuscles, Merkel disk receptors, and tactile corpuscles. All of these receptors stimulate an action potential in afferent nerves found in various layers of the skin and body. The afferent neuron travels to the spinal column and then to the brain where the information is processed. Damage to the peripheral nervous system or central nervous system can result in a decline or loss of pallesthesia.
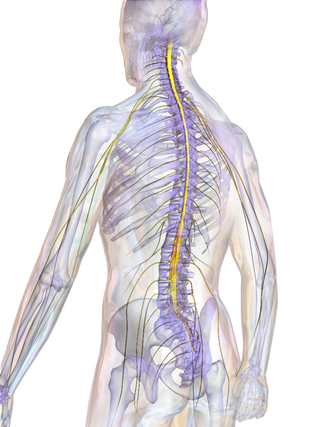
The spinal cord is a long, thin, tubular structure made up of nervous tissue that extends from the medulla oblongata in the lower brainstem to the lumbar region of the vertebral column (backbone) of vertebrate animals. The center of the spinal cord is hollow and contains a structure called the central canal, which contains cerebrospinal fluid. The spinal cord is also covered by meninges and enclosed by the neural arches. Together, the brain and spinal cord make up the central nervous system.
In neuroscience, the N100 or N1 is a large, negative-going evoked potential measured by electroencephalography ; it peaks in adults between 80 and 120 milliseconds after the onset of a stimulus, and is distributed mostly over the fronto-central region of the scalp. It is elicited by any unpredictable stimulus in the absence of task demands. It is often referred to with the following P200 evoked potential as the "N100-P200" or "N1-P2" complex. While most research focuses on auditory stimuli, the N100 also occurs for visual, olfactory, heat, pain, balance, respiration blocking, and somatosensory stimuli.













