
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom bonded to two organyl groups. They have the general formula R−O−R′, where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

Ethylene is a hydrocarbon which has the formula C2H4 or H2C=CH2. It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resultant aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and pharmaceuticals. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundations of many chemical industries.

1-Hexene (hex-1-ene) is an organic compound with the formula C6H12. It is an alkene that is classified in industry as higher olefin and an alpha-olefin, the latter term meaning that the double bond is located at the alpha (primary) position, endowing the compound with higher reactivity and thus useful chemical properties. 1-Hexene is an industrially significant linear alpha olefin. 1-Hexene is a colourless liquid.

In organic chemistry, terminal alkenes are a family of organic compounds which are alkenes with a chemical formula CxH2x, distinguished by having a double bond at the primary, alpha (α), or 1- position. This location of a double bond enhances the reactivity of the compound and makes it useful for a number of applications.

1-Octene is an organic compound with a formula CH2CHC6H13. The alkene is classified as a higher olefin and alpha-olefin, meaning that the double bond is located at the alpha (primary) position, endowing this compound with higher reactivity and thus useful chemical properties. 1-Octene is one of the important linear alpha olefins in industry. It is a colourless liquid.
The Shell higher olefin process (SHOP) is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell. The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors plasticizers and detergents. The annual global production of olefines through this method is over one million tonnes.

Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer triisobutylaluminium, and the titanium-aluminium compound called Tebbe's reagent. The behavior of organoaluminium compounds can be understood in terms of the polarity of the C−Al bond and the high Lewis acidity of the three-coordinated species. Industrially, these compounds are mainly used for the production of polyolefins.
Oxo alcohols are alcohols that are prepared by adding carbon monoxide (CO) and hydrogen to an olefin to obtain an aldehyde using the hydroformylation reaction and then hydrogenating the aldehyde to obtain the alcohol. An intermediate step of adding two aldehydes together to obtain a larger aldehyde can precede the hydrogenation. Long chain oxo-alcohols are often prepared using alpha-olefins from the Shell higher olefin process, to give secondary alcohols such as isodecyl alcohol.
In organometallic chemistry, a migratory insertion is a type of reaction wherein two ligands on a metal complex combine. It is a subset of reactions that very closely resembles the insertion reactions, and both are differentiated by the mechanism that leads to the resulting stereochemistry of the products. However, often the two are used interchangeably because the mechanism is sometimes unknown. Therefore, migratory insertion reactions or insertion reactions, for short, are defined not by the mechanism but by the overall regiochemistry wherein one chemical entity interposes itself into an existing bond of typically a second chemical entity e.g.:
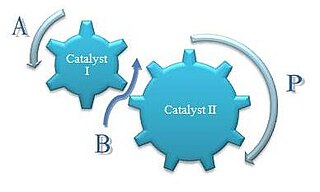
Concurrent tandem catalysis (CTC) is a technique in chemistry where multiple catalysts produce a product otherwise not accessible by a single catalyst. It is usually practiced as homogeneous catalysis. Scheme 1 illustrates this process. Molecule A enters this catalytic system to produce the comonomer, B, which along with A enters the next catalytic process to produce the final product, P. This one-pot approach can decrease product loss from isolation or purification of intermediates. Reactions with relatively unstable products can be generated as intermediates because they are only transient species and are immediately used in a consecutive reaction.

Linear alkylbenzenes (sometimes also known as LABs) are a family of organic compounds with the formula C6H5CnH2n+1. Typically, n lies between 10 and 16, although generally supplied as a tighter cut, such as C12-C15, C12-C13 and C10-C13, for detergent use. The CnH2n+1 chain is unbranched. They are mainly produced as intermediate in the production of surfactants, for use in detergent. Since the 1960s, LABs have emerged as the dominant precursor of biodegradable detergents.
1-Dodecene is an alkene with the formula C10H21CH=CH2, consisting of a chain of twelve carbon atoms ending with a double bond. While there are many isomers of dodecene depending on which carbon the double bond is placed, this isomer is of greater commercial importance. It is classified as an alpha-olefin. Alpha-olefins are distinguished by having a double bond at the primary or alpha (α) position. This location of a double bond enhances the reactivity of the compound and makes it useful for a number of applications, especially for the production of detergents.
In organic chemistry, the Ziegler process is a method for producing fatty alcohols from ethylene using an organoaluminium compound. The reaction produces linear primary alcohols with an even numbered carbon chain. The process uses an aluminum compound to oligomerize ethylene and allow the resulting alkyl group to be oxygenated. The usually targeted products are fatty alcohols, which are otherwise derived from natural fats and oils. Fatty alcohols are used in food and chemical processing. They are useful due to their amphipathic nature. The synthesis route is named after Karl Ziegler, who described the process in 1955.
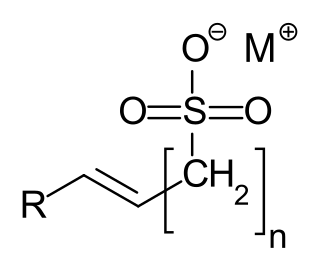
α-Olefin sulfonates are a group of anionic surfactants, which are used as detergents. The compounds contain a - mostly linear, primary - alkyl R and a monovalent cation M, preferably sodium. The most frequently used example of this group of substances is sodium α-olefin sulfonate.












