
Ethylene is a hydrocarbon which has the formula C2H4 or H2C=CH2. It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene.
In chemistry, a monomer is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.

Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including bottles, etc.). As of 2017, over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market.
Butene, also known as butylene, is an alkene with the formula C4H8. The word butene may refer to any of the individual compounds. They are colourless gases that are present in crude oil as a minor constituent in quantities that are too small for viable extraction. Butene is therefore obtained by catalytic cracking of long-chain hydrocarbons left during refining of crude oil. Cracking produces a mixture of products, and the butene is extracted from this by fractional distillation.
In organic chemistry, hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention: production capacity reached 6.6×106 tons in 1995. It is important because aldehydes are easily converted into many secondary products. For example, the resultant aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in speciality chemicals, relevant to the organic synthesis of fragrances and pharmaceuticals. The development of hydroformylation is one of the premier achievements of 20th-century industrial chemistry.
A post-metallocene catalyst is a kind of catalyst for the polymerization of olefins, i.e., the industrial production of some of the most common plastics. "Post-metallocene" refers to a class of homogeneous catalysts that are not metallocenes. This area has attracted much attention because the market for polyethylene, polypropylene, and related copolymers is large. There is a corresponding intense market for new processes as indicated by the fact that, in the US alone, 50,000 patents were issued between 1991-2007 on polyethylene and polypropylene.
The Fischer–Tropsch process (FT) is a collection of chemical reactions that converts a mixture of carbon monoxide and hydrogen, known as syngas, into liquid hydrocarbons. These reactions occur in the presence of metal catalysts, typically at temperatures of 150–300 °C (302–572 °F) and pressures of one to several tens of atmospheres. The Fischer–Tropsch process is an important reaction in both coal liquefaction and gas to liquids technology for producing liquid hydrocarbons.

Linear low-density polyethylene (LLDPE) is a substantially linear polymer (polyethylene), with significant numbers of short branches, commonly made by copolymerization of ethylene with longer-chain olefins. Linear low-density polyethylene differs structurally from conventional low-density polyethylene (LDPE) because of the absence of long chain branching. The linearity of LLDPE results from the different manufacturing processes of LLDPE and LDPE. In general, LLDPE is produced at lower temperatures and pressures by copolymerization of ethylene and such higher alpha-olefins as butene, hexene, or octene. The copolymerization process produces an LLDPE polymer that has a narrower molecular weight distribution than conventional LDPE and in combination with the linear structure, significantly different rheological properties.
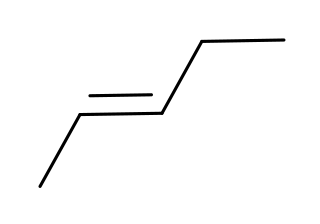
Pentenes are alkenes with the chemical formula C
5H
10. Each molecule contains one double bond within its molecular structure. Six different compounds are in this class, differing from each other by whether the carbon atoms are attached linearly or in a branched structure and whether the double bond has a cis or trans form.
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More specialized polyolefins include polyisobutylene and polymethylpentene. They are all colorless or white oils or solids. Many copolymers are known, such as polybutene, which derives from a mixture of different butene isomers. The name of each polyolefin indicates the olefin from which it is prepared; for example, polyethylene is derived from ethylene, and polymethylpentene is derived from 4-methyl-1-pentene. Polyolefins are not olefins themselves because the double bond of each olefin monomer is opened in order to form the polymer. Monomers having more than one double bond such as butadiene and isoprene yield polymers that contain double bonds (polybutadiene and polyisoprene) and are usually not considered polyolefins. Polyolefins are the foundations of many chemical industries.

1-Hexene (hex-1-ene) is an organic compound with the formula C6H12. It is an alkene that is classified in industry as higher olefin and an alpha-olefin, the latter term meaning that the double bond is located at the alpha (primary) position, endowing the compound with higher reactivity and thus useful chemical properties. 1-Hexene is an industrially significant linear alpha olefin. 1-Hexene is a colourless liquid.

In organic chemistry, terminal alkenes are a family of organic compounds which are alkenes with a chemical formula CxH2x, distinguished by having a double bond at the primary, alpha (α), or 1- position. This location of a double bond enhances the reactivity of the compound and makes it useful for a number of applications.

Straight-chain terminal alkenes, also called linear alpha olefins (LAO) or normal alpha olefins (NAO), are alkenes (olefins) having a chemical formula CnH2n, distinguished from other alkenes with a similar molecular formula by being terminal alkenes, in which the double bond occurs at the alpha position, and by having a linear (unbranched) hydrocarbon chain.

But-1-ene (or 1-butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas that is easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin (terminal alkene). It is one of the isomers of butene (butylene). It is a precursor to diverse products.
The Shell higher olefin process (SHOP) is a chemical process for the production of linear alpha olefins via ethylene oligomerization and olefin metathesis invented and exploited by Royal Dutch Shell. The olefin products are converted to fatty aldehydes and then to fatty alcohols, which are precursors plasticizers and detergents. The annual global production of olefines through this method is over one million tonnes.
Decene is an organic compound with the chemical formula C10H20. Decene contains a chain of ten carbon atoms with one double bond, making it an alkene. There are many isomers of decene depending on the position and geometry of the double bond. Dec-1-ene is the only isomer of industrial importance. As an alpha olefin, it is used as a comonomer in copolymers and is an intermediate in the production of epoxides, amines, oxo alcohols, synthetic lubricants, synthetic fatty acids and alkylated aromatics.
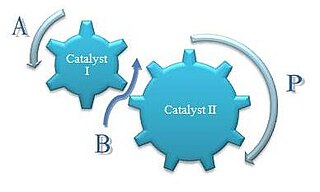
Concurrent tandem catalysis (CTC) is a technique in chemistry where multiple catalysts produce a product otherwise not accessible by a single catalyst. It is usually practiced as homogeneous catalysis. Scheme 1 illustrates this process. Molecule A enters this catalytic system to produce the comonomer, B, which along with A enters the next catalytic process to produce the final product, P. This one-pot approach can decrease product loss from isolation or purification of intermediates. Reactions with relatively unstable products can be generated as intermediates because they are only transient species and are immediately used in a consecutive reaction.
In organic chemistry, the Ziegler process is a method for producing fatty alcohols from ethylene using an organoaluminium compound. The reaction produces linear primary alcohols with an even numbered carbon chain. The process uses an aluminum compound to oligomerize ethylene and allow the resulting alkyl group to be oxygenated. The usually targeted products are fatty alcohols, which are otherwise derived from natural fats and oils. Fatty alcohols are used in food and chemical processing. They are useful due to their amphipathic nature. The synthesis route is named after Karl Ziegler, who described the process in 1955.










