In biology and biochemistry, protease inhibitors, or antiproteases, are molecules that inhibit the function of proteases. Many naturally occurring protease inhibitors are proteins.

DD-transpeptidase is a bacterial enzyme that catalyzes the transfer of the R-L-aca-D-alanyl moiety of R-L-aca-D-alanyl-D-alanine carbonyl donors to the γ-OH of their active-site serine and from this to a final acceptor. It is involved in bacterial cell wall biosynthesis, namely, the transpeptidation that crosslinks the peptide side chains of peptidoglycan strands.

A carboxypeptidase is a protease enzyme that hydrolyzes (cleaves) a peptide bond at the carboxy-terminal (C-terminal) end of a protein or peptide. This is in contrast to an aminopeptidases, which cleave peptide bonds at the N-terminus of proteins. Humans, animals, bacteria and plants contain several types of carboxypeptidases that have diverse functions ranging from catabolism to protein maturation. At least two mechanisms have been discussed.
Carboxypeptidase U is an enzyme. This enzyme catalyses the following chemical reaction

Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme (EC 2.1.2.1) which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate (THF) to 5,10-Methylenetetrahydrofolate (5,10-CH2-THF). This reaction provides the largest part of the one-carbon units available to the cell.
The discovery of an orally inactive peptide from snake venom established the important role of angiotensin converting enzyme (ACE) inhibitors in regulating blood pressure. This led to the development of Captopril, the first ACE inhibitor. When the adverse effects of Captopril became apparent new derivates were designed. Then after the discovery of two active sites of ACE: N-domain and C-domain, the development of domain-specific ACE inhibitors began.

Proto-oncogene serine/threonine-protein kinase Pim-1 is an enzyme that in humans is encoded by the PIM1 gene.

Aquayamycin is an anthraquinone derivative. It is an inhibitor of the enzyme tyrosine hydroxylase.
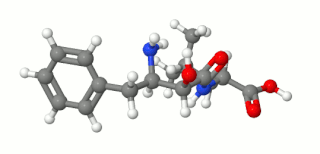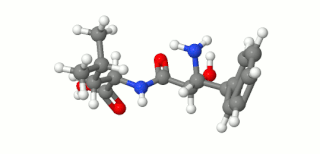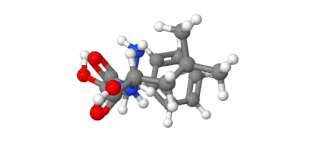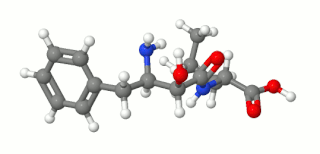
Ubenimex (INN), also known more commonly as bestatin, is a competitive, reversible protease inhibitor. It is an inhibitor of arginyl aminopeptidase (aminopeptidase B), leukotriene A4 hydrolase (a zinc metalloprotease that displays both epoxide hydrolase and aminopeptidase activities), alanyl aminopeptidase (aminopeptidase M/N), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase), and membrane dipeptidase (leukotriene D4 hydrolase). It is being studied for use in the treatment of acute myelocytic leukemia and lymphedema. It is derived from Streptomyces olivoreticuli. Ubenimex has been found to inhibit the enzymatic degradation of oxytocin, vasopressin, enkephalins, and various other peptides and compounds.
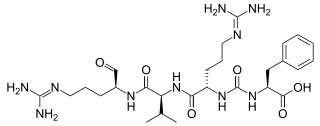
Antipain is an oligopeptide that is isolated from actinomycetes and used in biochemical research as a protease inhibitor of trypsin and papain. It was discovered in 1972 and was the first natural peptide found that contained an ureylene group. Antipain can aid in prevention of coagulation in blood. It is an inhibitor of serine and cysteine proteases.
Streptomyces amakusaensis is a bacterium species from the genus of Streptomyces which has been isolated from soil from the Amakusa Island in Japan. Streptomyces amakusaensis produces tuberin and nagstatin.
Streptomyces coeruleorubidus is a bacterium species from the genus of Streptomyces which has been isolated from marine sediment. Streptomyces coeruleorubidus produces the following medications: pacidamycin 1, baumycin B1, baumycin B2, baumycin C1, feudomycin A, feudomycin B, feudomycin C, ficellomycin, feudomycinone A, and rubomycin.
Streptomyces diastatochromogenes is a bacterium species from the genus of Streptomyces. Streptomyces diastatochromogenes produces polyketomycin, concanamycin A, concanamycin B, concanamycin C, momofulvenone A, azdimycin, toyocamycin and oligomycins.
Streptomyces griseoaurantiacus is a thermotolerant bacterium species from the genus of Streptomyces which was isolated from marine sediment. Streptomyces griseoaurantiacus produces the antibiotics manumycin, diperamycin and chinikomycin, and griseolic acid.
Streptomyces griseosporeus is a bacterium species from the genus of Streptomyces. Streptomyces griseosporeus produces taitomycin, 2-amino-4-hydroxypentanonic acid and liposidomycins.
Streptomyces melanosporofaciens is a bacterium species from the genus of Streptomyces which has been isolated from soil in Italy. Streptomyces melanosporofaciens produces elaiophylin, cyclooctatin, geldanamycin, chilaphylin and melanosporin. A mutant of Streptomyces melanosporofaciens has the ability to protect potatoes from common scab.
Streptomyces nitrosporeus is a bacterium species from the genus of Streptomyces which has been isolated from garden soil in Japan. Streptomyces nitrosporeus produces Benzastatin E, Benzastatin F, Benzastatin G Nitrosporeusine A and Nitrosporeusine B and the antibiotics nitrosporin and virantomycin and the inhibitor of angiotensin-converting enzyme foroxymithine. Streptomyces nitrosporeus can degrade cellulose.
Fostriecin is a type I polyketide synthase (PKS) derived natural product, originally isolated from the soil bacterium Streptomyces pulveraceus. It belongs to a class of natural products which characteristically contain a phosphate ester, an α,β-unsaturated lactam and a conjugated linear diene or triene chain produced by Streptomyces. This class includes structurally related compounds cytostatin and phoslactomycin. Fostriecin is a known potent and selective inhibitor of protein serine/threonine phosphatases, as well as DNA topoisomerase II. Due to its activity against protein phosphatases PP2A and PP4 which play a vital role in cell growth, cell division, and signal transduction, fostriecin was looked into for its antitumor activity in vivo and showed in vitro activity against leukemia, lung cancer, breast cancer, and ovarian cancer. This activity is thought to be due to PP2A's assumed role in regulating apoptosis of cells by activating cytotoxic T-lymphocytes and natural killer cells involved in tumor surveillance, along with human immunodeficiency virus-1 (HIV-1) transcription and replication.

Nagstatin is a strong competitive inhibitor of the N-acetyl-β-d-glucosaminidase with the molecular formula C12H17N3O6. Nagstatin is produced by the bacterium Streptomyces amakusaensis.
Tautomycetin is a natural product first isolated from Streptomyces griseochromogenes, a bacterium found in the soil of the Zhejiang Province, China. It was also later found in Penicillium urticae. It is a linear polyketide very similar in structure to tautomycin, both of which contain a unique dialkylmaleic anhydride moiety, which is essential for their pharmacological activity. Tautomycetin is a selective inhibitor of protein phosphatase 1.







