
Phagocytes are cells that protect the body by ingesting harmful foreign particles, bacteria, and dead or dying cells. Their name comes from the Greek phagein, "to eat" or "devour", and "-cyte", the suffix in biology denoting "cell", from the Greek kutos, "hollow vessel". They are essential for fighting infections and for subsequent immunity. Phagocytes are important throughout the animal kingdom and are highly developed within vertebrates. One litre of human blood contains about six billion phagocytes. They were discovered in 1882 by Ilya Ilyich Mechnikov while he was studying starfish larvae. Mechnikov was awarded the 1908 Nobel Prize in Physiology or Medicine for his discovery. Phagocytes occur in many species; some amoebae behave like macrophage phagocytes, which suggests that phagocytes appeared early in the evolution of life.

Cryptococcus neoformans is an encapsulated yeast and an obligate aerobe that can live in both plants and animals. Its teleomorph is Filobasidiella neoformans, a filamentous fungus belonging to the class Tremellomycetes. It is often found in bird excrement. Cryptococcus neoformans is an encapsulated fungal organism and it can cause disease in apparently immunocompetent, as well as immunocompromised, hosts.
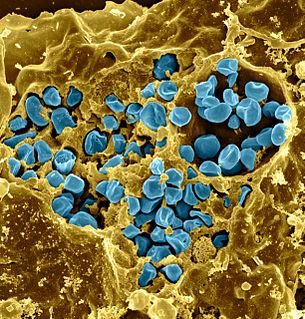
Francisella tularensis is a pathogenic species of Gram-negative coccobacillus, an aerobic bacterium. It is nonspore-forming, nonmotile, and the causative agent of tularemia, the pneumonic form of which is often lethal without treatment. It is a fastidious, facultative intracellular bacterium, which requires cysteine for growth. Due to its low infectious dose, ease of spread by aerosol, and high virulence, F. tularensis is classified as a Tier 1 Select Agent by the U.S. government, along with other potential agents of bioterrorism such as Yersinia pestis, Bacillus anthracis, and Ebola virus. When found in nature, Francisella tularensis can survive for several weeks at low temperatures in animal carcasses, soil, and water. In the laboratory, F. tularensis appears as small rods, and is grown best at 35-37°C.

Chlamydia psittaci is a lethal intracellular bacterial species that may cause endemic avian chlamydiosis, epizootic outbreaks in mammals, and respiratory psittacosis in humans. Potential hosts include feral birds and domesticated poultry, as well as cattle, pigs, sheep, and horses. C. psittaci is transmitted by inhalation, contact, or ingestion among birds and to mammals. Psittacosis in birds and in humans often starts with flu-like symptoms and becomes a life-threatening pneumonia. Many strains remain quiescent in birds until activated by stress. Birds are excellent, highly mobile vectors for the distribution of chlamydia infection, because they feed on, and have access to, the detritus of infected animals of all sorts.

Brucella is a genus of Gram-negative bacteria, named after David Bruce (1855–1931). They are small, nonencapsulated, nonmotile, facultatively intracellular coccobacilli.

Brucella melitensis is a Gram-negative coccobacillus bacterium from the Brucellaceae family. The bacterium causes ovine brucellosis, along with Brucella ovis. It can infect sheep, cattle, and sometimes humans, and it can be transmitted by the stable fly. It is zoonotic, unlike B. ovis, causing Malta fever or localized brucellosis in humans.
Intracellular parasites are microparasites that are capable of growing and reproducing inside the cells of a host. Some parasites can cause disease.

Bernhard Lauritz Frederik Bang, was a Danish veterinarian. He discovered Brucella abortus in 1897, which came to be known as Bang's bacillus. Bang's bacillus was the cause of the contagious Bang's disease which can cause pregnant cattle to abort, and causes undulant fever in humans.
Brucella abortus is a Gram-negative proteobacterium in the family Brucellaceae and is one of the causative agents of brucellosis. The rod-shaped pathogen is classified under the domain Bacteria. The prokaryotic B. abortus is non-spore-forming, nonmotile and aerobic.

In biology, a phagolysosome, or endolysosome, is a cytoplasmic body formed by the fusion of a phagosome with a lysosome in a process that occurs during phagocytosis. Formation of phagolysosomes is essential for the intracellular destruction of microorganisms and pathogens. It takes place when the phagosome's and lysosome's membranes 'collide', at which point the lysosomal contents—including hydrolytic enzymes—are discharged into the phagosome in an explosive manner and digest the particles that the phagosome had ingested. Some products of the digestion are useful materials and are moved into the cytoplasm; others are exported by exocytosis.
Listeriolysin O (LLO) is a hemolysin produced by the bacterium Listeria monocytogenes, the pathogen responsible for causing listeriosis. The toxin may be considered a virulence factor, since it is crucial for the virulence of L. monocytogenes.

Rhodococcus equi is a Gram-positive coccobacillus bacterium. The organism is commonly found in dry and dusty soil and can be important for diseases of domesticated animals. The frequency of infection can reach near 60%. R. equi is an important pathogen causing pneumonia in foals. Since 2008, R. equi has been known to infect wild boar and domestic pigs. R. equi can infect humans. At-risk groups are immunocompromised people, such as HIV-AIDS patients or transplant recipients. Rhodococcus infection in these patients resemble clinical and pathological signs of pulmonary tuberculosis. It is facultative intracellular.
The alpha-D-phosphohexomutases are a large superfamily of enzymes, with members in all three domains of life. Enzymes from this superfamily are ubiquitous in organisms from E. Coli to humans, and catalyze a phosphoryl transfer reaction on a phosphosugar substrate. Four well studied subgroups in the superfamily are:
- Phosphoglucomutase (PGM)
- Phosphoglucomutase/Phosphomannomutase (PGM/PMM)
- Phosphoglucosamine mutase (PNGM)
- Phosphoaceytlglucosamine mutase (PAGM)
Actinobacillus pleuropneumoniae, is a Gram-negative, facultative anaerobic, respiratory pathogen found in pigs. It was first reported in 1957, and was formally declared to be the causative agent of porcine pleuropneumonia in 1964. It was reclassified in 1983 after DNA studies showed it was more closely related to A. lignieresii.
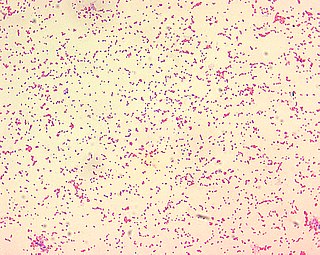
Brucella ceti is a gram negative bacterial pathogen of the Brucellaceae family that causes brucellosis in cetaceans. Brucella ceti has been found in both classes of cetaceans, mysticetes and odontocetes. Brucellosis in some dolphins and porpoises can result in serious clinical signs including fetal abortions, male infertility, neurobrucellosis, cardiopathies, bone and skin lesions, strand events, and death.
Brucella pinnipedialis is a species of bacteria. It causes infections and related diseases primarily in pinnipeds and cetaceans.

Vomocytosis is the cellular process by which live organisms that have previously been engulfed by a white blood cell are expelled without being destroyed. Vomocytosis was first reported in 2006 by two groups, working simultaneously in the UK and the USA, based on time-lapse microscopy footage characterising the interaction between macrophages and the human fungal pathogen Cryptococcus neoformans. Subsequently, this process has also been seen with other fungal pathogens such as Candida albicans and Candida krusei. It has also been speculated that the process may be related to the expulsion of bacterial pathogens such as Mycobacterium marinum from host cells. Vomocytosis has been observed in phagocytic cells from mice, humans and birds, as well as being directly observed in zebrafish and indirectly detected in mice. Amoebae exhibit a similar process to vomocytosis whereby phagosomal material that cannot be digested is exocytosed. Cryptococci are exocytosed from amoebae via this mechanism but inhibition of the constitutive pathway demonstrated that cryptococci could also be expelled via vomocytosis.
Bacterial small RNAs (sRNA) are an important class of regulatory molecules in bacteria such as Brucella. They are often bound to the chaperone protein Hfq, which allows them to interact with mRNA(s). In Brucella suis 1330 RNA sequencing identified a novel list of 33 sRNAs and 62 Hfq-associated mRNAs. In Brucella melitensis eight novel sRNA genes were identified using bioinformatic and experimental approach. One of them BSR0602 was found to modulate the intracellular survival of B. melitensis. In another large-scale deep sequencing study 1321 sRNAs were identified in B. melitensis. BSR0441 sRNA was further investigated in this study and shown to play role in the intracellular survival. sRNA BM-sr0117 from Brucella melitensis was identified and shown to be bound to and cleaved by Bm-RNase III. AbcR and AbcR2 were studied B. abortus. Seven novel sRNAs were validated and their interaction with a putative target sequence was verified in B. abortus.

















